Adolescent idiopathic scoliosis (AIS) is the most common type of scoliosis, affecting children from the age of 10.1 In addition to back pain, poor self-perceived health, reduced social participation and cosmetic issues, severe thoracic curves are also strongly associated with reduced pulmonary function.1 When the scoliosis is severe (Cobb angles above 45–50°) and conservative treatment is not sufficient, surgical management is considered to correct the spinal deformity, maintain/enhance pulmonary function, minimize pain and morbidity.2 Previous studies assessing the impact of posterior spinal fusion in AIS patients have showed that pulmonary function improved,3 decreased,4 or remained unchanged5 after surgery. In general, the studies did not assess respiratory muscle strength, which could also be impaired due to the biomechanics changes of the spine and thoracic cage.6 Therefore, this study aimed to evaluate pulmonary function and respiratory muscle strength three months after corrective surgery for AIS.
From November 2012 to May 2013, 12 Caucasian females with AIS (15.1±1.6 years of age; height: 158±13cm; weight: 51.5±10.5kg; body mass index: 20.2±2.2kg/m2) were enrolled in this prospective study. AIS group was sequentially recruited in the Paediatrics Services, Centro Hospitalar Porto, Portugal. Inclusion criteria: diagnosis of AIS, aged 10 or over, thoracic Cobb angle ≥40°. Exclusion criteria: bronchial asthma and other pulmonary, cardiovascular or skeletal muscle problems, and previous spinal surgery. Study procedures followed the ethical standards on human experimentation. Written informed consent was obtained from parents/guardians. Ethics Committee of the Centro Hospitalar do Porto approved the study. Pulmonary function and respiratory muscle strength were obtained preoperatively and at 3 months’ follow-up. Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF) and the fraction of FVC expired in one second (FEV1.FVC%) were assessed using a computerized spirometer (Spirolab III, MIR Medical International Research, Roma, Italy), following the ATS/ERS standards7; predicted values (%) were calculated using the global lung function 2012 equations,8 with the exception of PEF.9 Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) muscle strength were measured with a digital mouth pressure meter (Micro Respiratory Muscle Analyzer, CareFusion, Basingstoke, UK). All patients underwent posterior spinal fusion with autologous bone graft. Fusion levels ranged from T2 (most proximal) to L4 (most distal). Average preoperative thoracic coronal Cobb measurement was 54.9±8.9° (ranged from 42° to 62°). Data were analyzed using SPSS 17.0. Normality of data distribution was tested with the Shapiro–Wilk test. Data were not normally distributed. Differences in the AIS between pre and post-surgery values were tested with the Wilcoxon signed-rank test. Level of significance was set as p≤0.05.
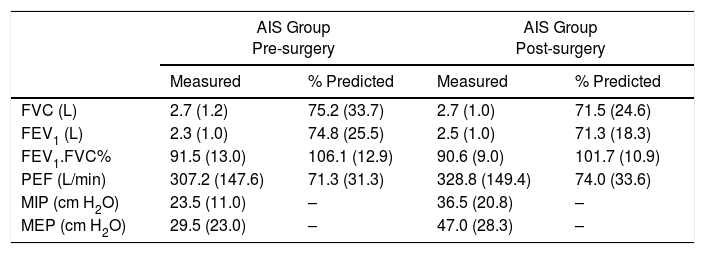
The results showed that at 3 months after surgery the pulmonary function was not significantly improved compared to the pre-surgery values; FVC and FEV1 remained approximately 30% below their predictive values (Table 1). With respect to the respiratory muscle strength, MEP increased significantly from pre-surgery to 3 months’ post-surgery (p=0.045), whereas MIP did not increase significantly (p=0.092) (Table 1).
Comparison of spirometric and respiratory muscle strength values between pre-surgery and 3 months post-surgery [median (interquartile range)].
| AIS Group Pre-surgery | AIS Group Post-surgery | |||
|---|---|---|---|---|
| Measured | % Predicted | Measured | % Predicted | |
| FVC (L) | 2.7 (1.2) | 75.2 (33.7) | 2.7 (1.0) | 71.5 (24.6) |
| FEV1 (L) | 2.3 (1.0) | 74.8 (25.5) | 2.5 (1.0) | 71.3 (18.3) |
| FEV1.FVC% | 91.5 (13.0) | 106.1 (12.9) | 90.6 (9.0) | 101.7 (10.9) |
| PEF (L/min) | 307.2 (147.6) | 71.3 (31.3) | 328.8 (149.4) | 74.0 (33.6) |
| MIP (cm H2O) | 23.5 (11.0) | – | 36.5 (20.8) | – |
| MEP (cm H2O) | 29.5 (23.0) | – | 47.0 (28.3) | – |
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEV1.FVC%, the fraction of FVC expired in one second; PEF, peak expiratory flow; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure.
Our results in short term (3 months after surgery) are similar to others which showed no significant changes in lung function in the first months after this surgical correction.5 A recent systemic review and meta-analysis10 encompassing eleven studies that assessed the effect of posterior spinal fusion on pulmonary function in patients with AIS, concluded that this procedure promotes small improvements in pulmonary function at two years’ post-surgery. However, in long term (from 6.9 to 20 years after surgery), this meta-analysis showed moderate improvements in FEV1 and forced vital capacity when compared to baseline. The correction of the chest deformity leads to better chest wall mechanics, which in turn improves the mechanical efficiency of the respiratory muscles, hence improving the ability to generate higher MIP and MEP.11 This study has some limitations, one of which is the small sample size; also, the three-month follow up period may not be enough to detect significant improvements in pulmonary function; lastly post-surgery complications or physiotherapy treatments received after hospital discharge were not taken into account. Future studies should increase the follow-up period and consider additional variables, such as quality of life, self-esteem, level of physical activity and exercise participation. In conclusion, (i) patients with AIS at 3 months after surgery showed a pulmonary function similar to preoperative values, i.e. our results suggest that the posterior spinal fusion with autologous bone graft does not adversely affect pulmonary function at 3 months post-surgery; (ii) patients showed an increase in expiratory muscle strength at 3 months after surgery.
Conflicts of interestThe authors have no conflicts of interest to declare.