Omalizumab is a monoclonal anti-IgE antibody approved for the treatment of uncontrolled severe persistent allergic asthma.1 Although several studies have shown its benefits,2–4 the optimal duration of the therapy and the effect after its withdrawal remains unknown.
We performed a study to assess the clinical effect of Omalizumab discontinuation, in patients with severe asthma. A current re-evaluation was conducted through questionnaires of asthma control, respiratory function tests and assessment on exacerbations.
Ten patients were included, all women, with a mean age of 49.5 years. All patients responded to Omalizumab therapy according to the GETE scale (Global Effectiveness Treatment Evaluation). The average time of treatment was 43.4±21.7 months (min. 9, max. 73). Omalizumab was stopped in 7 patients for clinical stability, and in the other 3 for complications (1 stroke, 1 breast cancer and another for severe anaemia). The median time from discontinuation of therapy was 28±25.3 months (min. 5, max. 76).
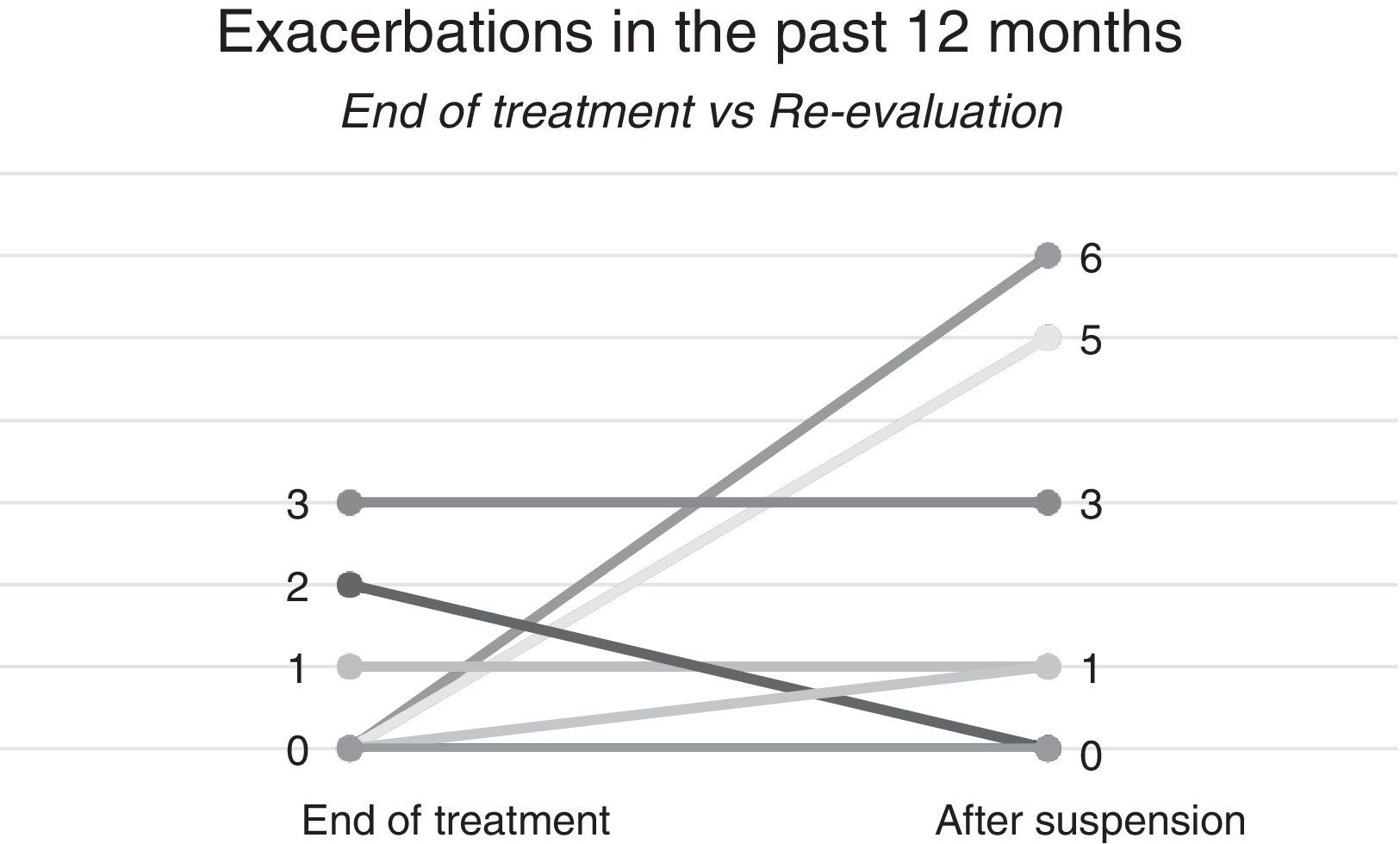
In our re-evaluation, the mean Control Allergic Rhinitis and Asthma test (CARAT) decreased from 21.6±6.6 to 19.9±7.8 and mean fractional exhaled nitric oxide (FeNO) value increased from 28.3±11.1 to 29.03±29.9ppb. Forced expiratory volume in 1s (FEV1) increased from 60.5±20.7% to 60.8±18.4%. The number of exacerbations within the last 12 months increased from 0.6±1.1 to 2.1±2.4 (Fig. 1).
Individual analysis showed a decrease in asthma control in 3 cases (30%), an increase in exacerbations was also observed in 4 patients (40%), and none of them had had exacerbations over the past 12 months at the end of treatment. The mean time to the first exacerbation was 6±5.1 months, and three patients had an exacerbation in the first 6 months. Two of these patients had been the most severe patients at the beginning based on ACT scores; they were under treatment for variable lengths of time (25/42/52 months).
During the 7 months follow up period, Omalizumab has already been reintroduced in one patient.
In the literature, there are only a few evaluations of patients following suspension of Omalizumab, with different study designs and inconsistent results.
The biggest report is from Nopp et al.5 who published a 1 long-term study (n=18) with a 3-year period of observation after Omalizumab suspension, where one third of the patients lost their asthma control. Molimard et al.6 performed a retrospective observational study in severe asthmatic patients (n=61) after discontinuation of Omalizumab therapy and loss of control was observed in 34 patients (55.7%); Omalizumab was reintroduced in 20 out of these 34 patients, but 20% of them became non-responders despite previous sensitivity.
Looking at our patients and at the studies described, it seems reasonable to maintain Omalizumab (beyond the standard period of 5 years7)/to reintroduce it in patients with frequent exacerbations who achieved control after Omalizumab treatment.
Besides the possibility of losing asthma control, there is a risk of secondary resistance to Omalizumab.6
Therefore, decisions on cessation of Omalizumab treatment should always be undertaken individually after weighing all the benefits and risks.
Conflicts of interestThe authors have no conflicts of interest to declare.