Tuberculosis (TB) in migrants represents an important clinical and public health threat, particularly in low TB incidence countries. The current review is aimed to assess issues related to screening and treatment of migrants with latent TB infection or TB disease.
Tuberculosis (TB) is a clinical and public health issue worldwide. Its incidence is significant in vulnerable population groups, particularly in immunocompromised patients and in people living in disadvantaged regions or settings.
The annual incidence rate is declining at a slow rate, considerably lower than the rate it was expected to have achieved by the End TB Strategy goals.1
In 2015, 60,195 cases of TB were reported in 30 European Union/European Economic Area (EU/EEA) countries. A significant proportion of EU cases is represented by patients of foreign origin.2
Following economic and political crises in Africa and in the Middle East a dramatic flow of migrants and refugees has been recorded in the last decade, increasing the potential of TB burden in low TB incidence countries. Several authors and policy-makers have underscored the need to implement or scale-up a screening system for latent TB infection and TB disease to reduce the burden of disease and the probability of Mycobacterium tuberculosis in a low incidence setting. However, transmission of M. tuberculosis strains from migrants to the native population has been proved to be limited owing to poor social integration in the majority of the EU/EEA countries.3
Effective cross-border cooperation is needed to achieve appropriate clinical and public health management.
Several tools are available to help countries with cross-border TB activities, such as the minimum package for cross-border TB control and care prepared by the WHO European Region, as well as the cross-border case management of the ERS TB Consilium electronic platform.4,5
In 2015, 2.7 million migrants from EU/EEA non-member countries were estimated in the EU states.6
At risk migrants who should be screenedEven in populations where TB is considered common, the vast majority of individuals do not have the disease. As a consequence, any programme aimed at detecting TB in a population group in which the disease is expected to be more prevalent than in a reference population, for instance in migrant populations compared with the resident population of a country of settlement, has to accept unnecessary screening of a large number of individuals to detect those who really have the disease.
In many countries, a decision has been taken (mostly based on historical background, political decisions and fear of transmission of contagious disease to the resident population) to screen all migrants from foreign countries for communicable disease, mainly TB.7 As the yield of such unselective strategies is low and the cost-effectiveness is unfavourable, whatever the screening method used,8 some countries have moved from a strategy of indiscriminate screening towards a more selective strategy, taking into account the expected risk of TB in selected migrant groups. The advantage of such policy is that it restricts screening to the group with the highest risk expected, thus sparing costs of unnecessary screening of large number of individuals, but the disadvantage is that is neglects groups with low risk where some cases of TB may still be present. As there is no consensus on the optimal trade-off of sensitivity and specificity of screening procedures, it is not surprising that the strategies differ widely between European countries, which makes comparison of cost-effectiveness very difficult.9
Furthermore, many countries have a different screening strategy between legal migrants applying for a residency permit (like foreign workers) and refugees and asylum seekers. The most obvious way of selecting the migrant groups with the highest risk of TB and LTBI is to consider the incidence rate in the country of origin, as reported by the WHO, taking into account the uncertainty of reporting in some countries or regions with a weak or disorganized health system. Recent reports from Germany10 and The Netherlands11 have confirmed that this could save large amounts of time and money without greatly decreasing the yield of screening. Reports from Belgium have contested these findings and maintain that the limiting effect of restricting screening to some migrant groups based on expected risk may not be effective.12 For foreign workers originating from the European community, no screening is usually requested, as they are allowed to circulate freely between the countries and contribute very little to the prevalence of TB in Europe.13 For foreign workers of extra-European origin, who contribute much more to the global TB prevalence in Europe,14 screening procedures according to a pre-determined risk assessment is usually performed, frequently in the form of a pre-migration screening15,16 and the yield is correlated with the incidence in the country of origin.17
The evaluation of TB risk according to WHO reports may not reflect all aspects associated with the risk of disease. For instance, migrants may belong to a population group with a lower risk than the local population, as they are usually young, healthy and able to travel. On the other side, the travel conditions may increase the risk of contamination before arrival in the country of settlement (crowded living and travel conditions, frequent stays in camps, prisons or shelters). A study in Switzerland has documented that the prevalence of LTBI is about double in migrants who travelled by land or sea (which usually takes several weeks or months) than in migrants who could afford to fly directly from their country of origin to Europe.18
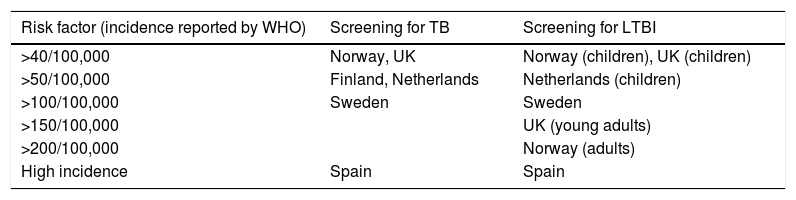
If the screening is performed according to a pre-selected risk definition, the cut-off is variable between countries in Europe (Table 1). Further differences exist in the implementation of a system for LTBI screening. An interesting model is used in Switzerland, where screening by symptoms is applied to all migrants but further confirmatory screening (by chest X-ray and sputum examination, if indicated) is performed only according to a scoring system based on the number of symptoms, the history of TB contacts and the origin of migrants. In this model, migrants with severe symptoms and those originating from regions with a very high TB incidence are all submitted to radiological screening, whereas others are examined only if the level of scoring combining the different information reaches a pre-determined value. An electronic version of the audio–visual screening system with automated scoring calculation in is available on www.tb-screen.ch.
Countries considering a risk factor (usually the incidence rate according to WHO in the country of origin) for implementing a selective screening for TB and LTBI. Other European countries screen all or no migrants for TB and LTBI, without considering an epidemiological risk factor (from Kunst et al.).9
| Risk factor (incidence reported by WHO) | Screening for TB | Screening for LTBI |
|---|---|---|
| >40/100,000 | Norway, UK | Norway (children), UK (children) |
| >50/100,000 | Finland, Netherlands | Netherlands (children) |
| >100/100,000 | Sweden | Sweden |
| >150/100,000 | UK (young adults) | |
| >200/100,000 | Norway (adults) | |
| High incidence | Spain | Spain |
The decision to screen migrants is seldom based on objective epidemiological considerations and there are no studies demonstrating the cost-effectiveness of systematic screening.19 Some evidence exists that selective screening of risk groups may be cost-effective,20 but the main issue is to ensure that all migrants, whatever their legal status and origin, have access to appropriate health care after entering in a new country.21
Evidence of diagnosis of LTBI and TB in migrantsTrends of migration flows continue to be predominantly from high-incidence to intermediate and low-incidence tuberculosis (TB) countries.22 The majority of migrants are people forced to migrate because of violence and poverty, and many of them arrive and live in the receiving country in poor conditions of health and quality of life.23 Consequently, immigrants have a higher prevalence of latent tuberculosis infection (LTBI) and active TB and they are at a dramatically higher risk of developing active disease than native-born populations either by progression of latent infection or by progression of a new infection acquired in the country of arrival.17,24–26
Although the impact of TB in immigrants on onward transmission towards native-born and notification rates does not seem significant in some countries,27,28 the early diagnosis of LTBI and active TB in immigrants is crucial for reducing the huge economic and non-economic burden caused by TB on this population and the health systems and a challenging need for TB elimination.29
Diagnosis of LTBI. Tuberculin skin test (TST) and interferon gamma release assays (IGRA) are used for screening LTBI in migrants before (pre-migration) or after (post-migration) the entry to the receiving country.23,30–35 Although IGRA have better specificity and some studies have shown that they could be more cost-effective than TST,20,24,36–38 robust evidence on reactivation rates after screening is required for defining what are the best test and strategy in each particular situation.20 In the meantime, TST remains the preferred test for screening LTBI in children 5 years old and younger or the alternative test to IGRA (according to availability and local cost) in adult migrants from high TB burden countries.39–41 TST (only or plus IGRA) is the most frequently used test for screening LTBI in European countries.42 In people who are in close contact with active TB cases or have high risk of progression from latent to active disease, a cutoff of ≥5mm should be used for defining a positive test regardless previous BCG vaccination.
Diagnosis of active TB. An unequivocal diagnosis of TB needs a positive culture or a specific molecular test for M. tuberculosis. For optimizing the diagnostic yield of these tests in migrants, it is necessary to preselect people using a high sensitivity test.20,43,44,16 Initial screening using chest X-ray (CXR) followed by a highly specific test as GeneXpert MTB/RIF or mycobacterial culture has the highest positive predictive value and lowest number needed to screen for detecting active TB in migrants.17,20,44 It would be better to use the symptom questionnaires as additional to the radiography and not as the only method because of its low sensitivity and specificity.20 Although smear microscopy has low sensitivity and a non-insignificant proportion of false positives, it is useful for the treatment decision because of its easy availability and low cost but it needs emphasizing that confirmatory culture or examination should be performed. In Europe, the majority of countries used systematic CXR only or with symptom-based questionnaires.42 The best algorithm for screening active TB may vary according to availability, cost and predictive values in each country.8
Evidence of therapy for TB and LTBI in migrantsThe WHO considers migrants as a population who should be screened and treated for both latent and active TB.29
In fact, screening for TB in migrant populations has been carried out for decades: its focus has been to protect the host community from imported TB cases by treating active cases and preventing the development of active tuberculosis.7
However, to accurately determine effectiveness of therapy for TB and LTBI in migrants, it would be necessary to know the same indicator used for non-migrant population. In the case of LTBI, we must at least know the yield of detection, diagnostic method used, percentage of individuals who started therapy and of those who completed it. Only then can we estimate how many active TB cases were prevented. For active TB, it would be important to know the screening methodology, the yield of diagnosis and the diagnostic methods used, percentage of individuals who started therapy and of those who completed the treatment and were considered either cured or as a failure. Unfortunately, most European countries do not regularly collect such information in migrant populations. Robust scientific evidence is missing; therefore, it is difficult to make a clear statement about how effective therapy of LTBI or active TB is in migrant populations.42,45
Besides, population mobility towards high-income countries has increased and that trend is expected to continue. Since most of the migrants come from low-income countries with high TB burden, that imposes new challenges for global TB control and eradication. There is great variability among countries in screening methods for latent or active TB. Added to this, migrant populations have different TB rates in their native countries and carry different comorbidities with them. The overload they impose on the health services in the host country could be overwhelming and very expensive. A thorough review of the current policies and their effectiveness to detect and treat both LTBI and active TB is needed.45,46
It has been estimated that TB among foreign-born people represents between 35% and 70% of all cases in low incidence countries. Most of the cases are due to reactivation of LTB which occurs soon after arrival, but the high rates of reactivation may last for more than 10 years.47–49 It is clear that migrant populations represent most of the TB cases in the EU, Canada and USA, jeopardizing the goal of TB elimination in those regions.42,45,46,48–50 Undocumented migrants are also a relevant problem since they cause 5–10% of the TB cases. In this group, data collection and compliance with any TB programme is inherently difficult.51 Also, in US, the number of multidrug-resistant TB cases in foreign-born persons is triple that of in U.S.-born persons, 1.2% and 0.4% respectively.48
It is considered that TB in migrants has a low overall public health impact in low-incidence countries, but the costs of treating migrants with active TB could be huge for the host country. In the USA, the estimated cost for the care of TB among the foreign-born accounts for more than $350 million per year. A similar figure is estimated in countries such as Canada and the UK.47
However, recently, the CDC from USA considered that treatment of LTBI in migrants coming from high TB burden countries is an effective approach to decreasing the number of reactivations and routine screening is standard practice.48
Despite the different conclusions from several studies, it is widely accepted that screening and treating latent and active TB in immigrants carries benefits for health and economy from a social point of view, especially if young migrants from high-burden countries are targeted. The threshold for “high burden” needs still to be defined.29,36 Also, targeting the screening to close contacts of active TB cases improves the cost-effectiveness to detect and prevent active TB cases.47
How migrants with active or latent TB should be treated is another concern. There is an agreement that treatment for active TB (sensitive or resistant) should be the same as for non-foreign-born, and most countries do that.36,47 Different treatments for latent TB have been used in several settings. Evidence exists for the efficacy and safety of 6-month isoniazid monotherapy, rifampicin monotherapy, and combination therapies with 3–4 months of isoniazid and rifampicin.52
The four-drug regimen (isoniazid, rifampin, ethambutol and pyrazinamide) for sensitive active TB is highly effective for non-migrant communities who comply with the treatment. Similar goals could be reached in migrant populations if a high rate of compliance was assured. If so, it seems very probable that any screening programme focused on detecting and treating LTBI and active TB would be cost-effective. The current problem is how and when to detect the candidate cases for preventive or curative therapy amongst the growing number of migrants and then, how to assure that they will comply with the treatments. Cost-effectiveness would be affected if any of the interlinked steps that precede the starting of therapy are not planned taking into account the several factors that make up the problem: the kind of migrants screened (age, burden of TB in their countries, comorbidities), the screening tools available in the host country and the additional work load and increases in costs for local health services.
Our knowledge regarding the effectiveness or cost-effectiveness of the therapies for TB and LTBI in migrants remains scarce and is limited to data related only to documented migrants. This group represents a minority of the foreign-born living in high-income countries. The undocumented group should also be included in any TB programme if TB elimination is really pursued.36,47
Pre- and post-arrival screening in migrantsGlobal policy environmentNew guidelines issued by the World Health Organization (WHO) in 2013 finally took a position on appropriateness and usefulness of screening for active TB as a programmatic control component. Although the guidelines did not specifically address screening migrants for active TB in low/intermediate burden settings they provide a clear blueprint for targeting well defined risk groups on the basis of baseline population prevalence and risk for progression towards active TB. The document does not provide specific guidance on the tools to be used in a screening programme but does highlight the need to maximize cost-effectiveness through targeted approaches.53
Similarly, with regards to Latent TB Screening (LTBI or TB infection screening) there is a lack of consolidated and migrant specific guidelines globally. However, in 2015, the WHO released the first ever guidelines for the diagnosis and management of LTBI. Recognizing the limitations in predicting progression towards active disease of currently available tools, namely Interferon Gamma Release Assays (IGRAS) and Tuberculin Skin Testing (TST).54
The current available body of evidence is therefore based on experiences at country level. In particular there is now a decennial body of literature summarizing and presenting data from low incidence countries, mainly focused on active TB screening from migrants from high and intermediate burden settings. Given the lack of common methodology and variability in the selection of measurable impact there is a substantial heterogeneity between studies, limiting the ability to analyze findings across settings and outcomes.
Active TB screening – tools and timingMost screening programmes for active TB are primarily designed to detect infectious and transmissible cases of TB and therefore focus on their ability to detect pulmonary TB (PTB). A combination of screening tools followed by a reflex confirmatory test is the usual common approach utilized by screening programmes. Symptoms screening along with immunodiagnostic testing and risk based assessment (i.e. country of origin) are the most common first line screening tools followed by confirmatory tests with molecular diagnostics (i.e. GeneXpert) and microbiological testing (culture increasingly favoured over smear microscopy).9,20
In relation to the timing of screening the approach can be defined at three different temporal stages namely pre-entry, at-entry and post-entry. These definitions relate to the timing of execution of a screening test vis-à-vis the crossing of a defined recipient country's border. While differentiating pre- and post-entry screening is generally straightforward, the definition of at-entry screening is more complex and is administrative rather than programmatic.
Pre-entry screeningPre-entry screening programmes aim to identify and eventually treat active TB cases before entry (or allow entry) to the recipient country. These programmes are traditionally tied to a mandatory visa submission and approval process. They are usually carried out in the country of origin (or host country in the case of refugee or asylum seeker).26
Mandatory TB screening at pre-entry stage records the highest coverage with percentages of execution among applicants close to 100%. However high coverage does not necessarily yield high numbers as high and low risk individuals are screened indiscriminately thus reducing the potential cost-effectiveness of screening.55
At entry screeningAs mentioned at-entry screening programmes are difficult to define temporally. Screening in these approaches is unlikely to occur at entry (i.e. upon presentation at the border) but rather reflect a referral system based on identification of risk upon presentation at the recipient countries’ borders.9,20,26,55
Post entry screeningPost-entry (also known as post arrival screening) are often a required component of access to specific services, for example registration with primary health service, job application or first point of contact with health delivery services. These programmes are usually targeted at specific country of origin and tend to have a variable yield depending on the approach and the level of access to post-entry services.9,20,26,55
Latent TB infection screeningCompared to the body of evidence available for active TB screening and in particular pulmonary tuberculosis, very little consolidated evidence exists on the execution and impact of LTBI screening. Published studies provide limited information about value of LTBI screening and no study has so far attempted to estimate the impact of TB prevention in LTBI screening programmes on overall incidence. Countries use different approaches with variable levels of follow-up in terms of duration and quality thus making an assessment of such approach not feasible at the moment.9,20
Conflicts of interestJean-Pierre Zellweger received horonaries for scientific conferences from Qiagen.