We conducted a meta-analysis of published literature to identify the correlation between leptin receptor gene polymorphisms and the risk of obstructive sleep apnea syndrome (OSAS).
MethodsFive different single nucleotide polymorphisms (SNPs) were studied. Only Gln223Arg and Pro1019Pro had multiple studies. Nine studies focused on the correlation between Gln223Arg and Pro1019Pro polymorphisms and OSAS risk. Fixed-effects model or random-effects model was used to calculate the pooled odds ratio (ORs) and its corresponding 95% confidence interval (95% CI). The Begg's, Egger's, Perter's and Harbord tests were used to measure publication bias. Sensitivity analysis was also performed to ensure the robustness of the findings.
ResultsSix studies on Gln223Arg polymorphisms (661 cases and 498 controls) and three studies on Pro1019Pro polymorphisms (561 cases and 561 controls) were extracted. There was no correlation between the leptin receptor Gln223Arg polymorphism and the risk of OSAS (odd ratio=0.86, 95% CI=0.68–1.10, P=0.23). However, Caucasian OSAS patients had a higher Arg allele frequency; whereas Chinese population with G genotype were more susceptible to OSAS (odd ratio=1.28, 95% CI=1.04–1.57, P=0.02) in the studies on Pro1019Pro polymorphisms.
ConclusionThe Gln223Arg polymorphisms in the Caucasian population and the Pro1019Pro polymorphisms in the Chinese population are risk factors for OSAS.
Obstructive sleep apnea syndrome (OSAS) is a prevalent sleep disorder characterized by recurrent episodes of partial or complete collapse of the upper airway during sleep, resulting in oxygen desaturation and sleep fragmentation. A report from the World Health Organization estimates that OSAS affects 5–20 million people in Europe.1 However, the etiology of OSAS is unclear. Previous studies indicate that leptin receptor may play an important role in the pathogenesis of OSAS.
Obesity is an important risk factor of OSAS.2,3 Leptin and leptin receptors contribute significantly to obesity.2–6 Plasma leptin levels are usually higher in patients with obesity. Leptin is a protein hormone secreted by adipose cells, especially white adipose cells. It can suppress appetite, reduce energy intake, increase energy expenditure and inhibit fat synthesis. Normally, a high level of leptin causes weight loss in healthy controls, but leptin does not work on obese people. This phenomenon is termed “leptin resistance”.4 In animal studies, genetically mutated mice lack functional leptin receptors are obese, although they have a higher level of plasma leptin.5,6 The results from animal studies imply that the leptin resistance caused by leptin receptor mutation may also exist in humans.
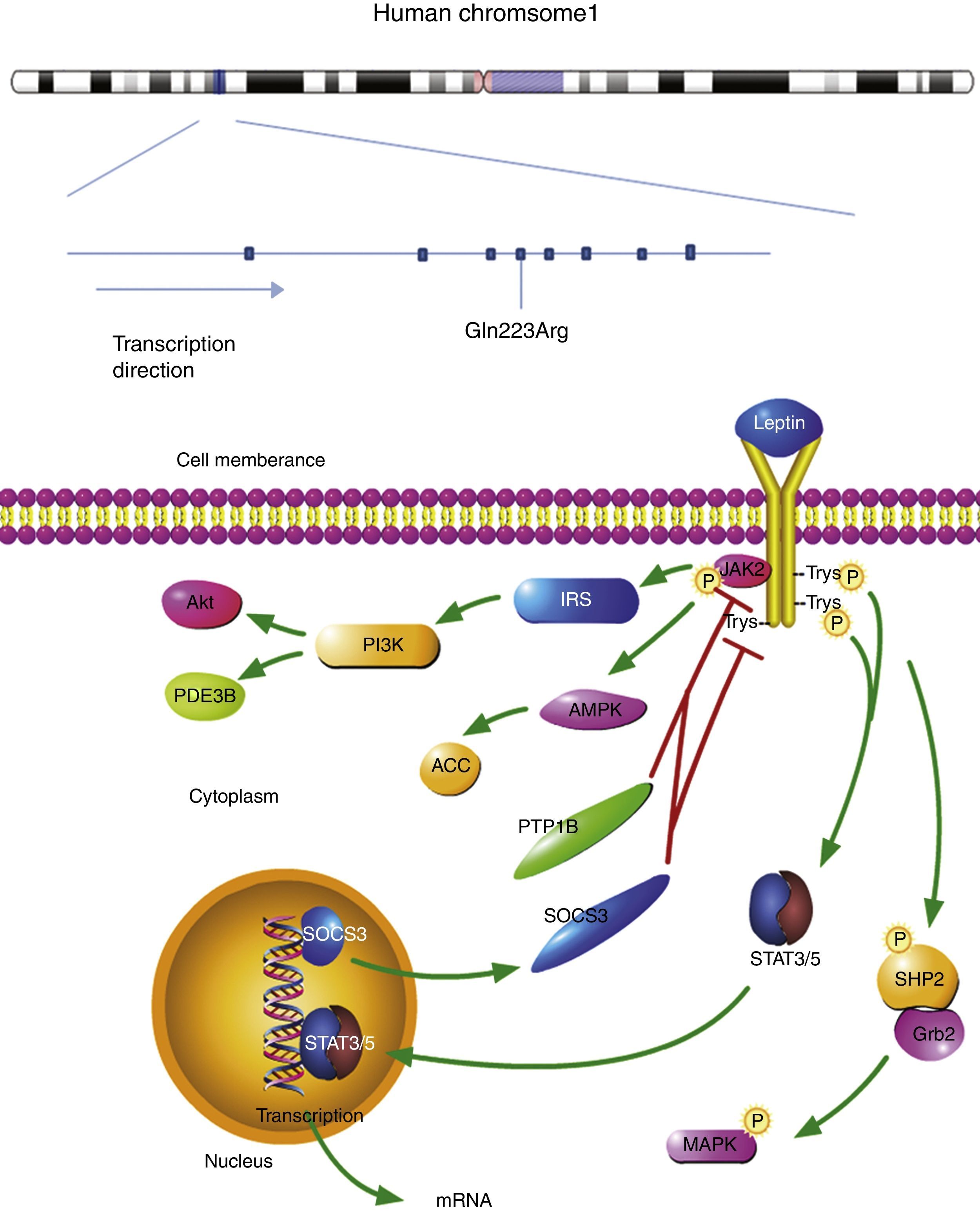
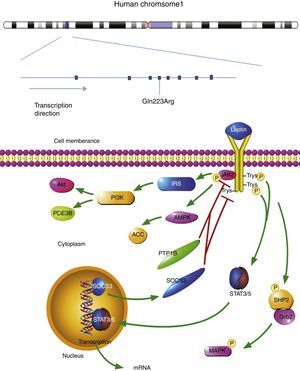
The plasma leptin binds to the homodimeric leptin receptors. The extracellular domain of leptin receptor contains 816 amino acid residues. The leptin-bond leptin receptor recruits Janus kinase 2 (JAK2) in its intracellular domain. The complex is activated by the phosphorylation of JAK2, Tyr985, Tyr1077, and Tyr1138. Phosphor-Tyr985 is recruited to SH2-containing protein tyrosine phosphatase 2 (SHP2), then binds to the adaptor protein growth factor receptor-bound protein 2 (Grb2). The mitogen-activated protein kinase (MAPK) is activated to initiate the signaling cascade. Leptin regulates metabolism through Janus kinase 2 (AMPK) and acetyl-CoA carboxylase (ACC) in hypothalamus and peripheral organs. At the same time, leptin receptor-JAK2 complex also activates phosphatidylinositol 3 kinase (PI3K) pathways. In this signaling pathway, insulin receptor substrate (IRS) is phosphorylated first, leading to PI3K activation. Phosphodiesterase 3B (PDE3B) is an important downstream target of PI3K in the leptin signaling pathway.7
Signal transducer and activator of transcription 3 (STAT3) and STAT5 bind to phospho-Tyr1138 and phospho-Tyr1077, respectively. The active STAT3 and STAT5 recruit another STAT3 and STAT5. The dimers are transported into the nucleus and begin the transcription of target genes. The anorexigenic effect is activated by this signaling pathway. Suppressor of cytokine signaling 3 (SOCS3), a target gene of STAT3, inhibits the JAK2/STAT3 pathway by interacting with phospho-Tyr985 or JAK2 and acting as a feedback inhibitor of leptin signaling pathway. The signaling pathway of leptin receptor is shown in Fig. 1.7
The location of leptin receptor SNPs and leptin receptor signaling pathway. The leptin bond leptin receptor recruits JAK2 and activates tyrosines. Then Phosphor-tyrosines recruits SH2, Grb2, STAT3, and STAT5, respectively. AMPK and ACC are activated to regulate metabolism. Leptin receptor-JAK2 complex also activates PI3K pathways: phosphorylated IRS leads to PI3K activation, following by activating PDE3B and Akt. A target gene of STAT3, SOCS3 mediates anorexigenic effect. SNP: single nucleotide polymorphism; JAK2: janus kinase 2; SHP2: SH2-containing protein tyrosine phosphatase 2; Grb2: growth factor receptor-bound protein 2; AMPK: 5′ adenosine monophosphate activated protein kinase; ACC: acetyl-CoA carboxylase; PI3K: phosphatidylinositol 3 kinase; IRS: insulin receptor substrate; PDE3B: phosphodiesterase 3B; Akt: also known as protein kinase B (pkb); SOC3: suppressor of cytokine signaling 3.
Previous studies show that there is a positive correlation between the serum leptin levels and obesity.8 Furthermore, the mutations in leptin receptor gene cause severe obesity in humans9 and the OSAS patients have a higher plasma leptin level.10 Therefore, the evidence may imply that the leptin receptor mutations may be associated with the risk of OSAS.
OSAS is a familial disease and is caused by the interaction between environmental and genetic factors.11 The leptin receptor gene is located on chromosome 1p31, and contains 20 exons and 19 introns. The total length of the leptin receptor gene is ∼70kb and is composed of 1165 amino acid. From a public health perspective, the current challenge is to identify the susceptibility gene and ascertain the cause of OSAS. Previous studies show that the mutation of melanocortin-4 receptor gene induces deficits in leptin-melanocortin pathway, which represents the genetic basis of obesity and OSAS. Other leptin receptor genes may also play important roles in the pathogenesis of this disorder. It is important to understand the association between leptin receptor polymorphisms and OSAS risk; therefore, individuals with higher genetic risk can be identified and receive targeted preventive therapy. We have conducted a systematic review of published literature on this topic and analyzed the correlations between leptin receptor polymorphisms and OSAS risk.
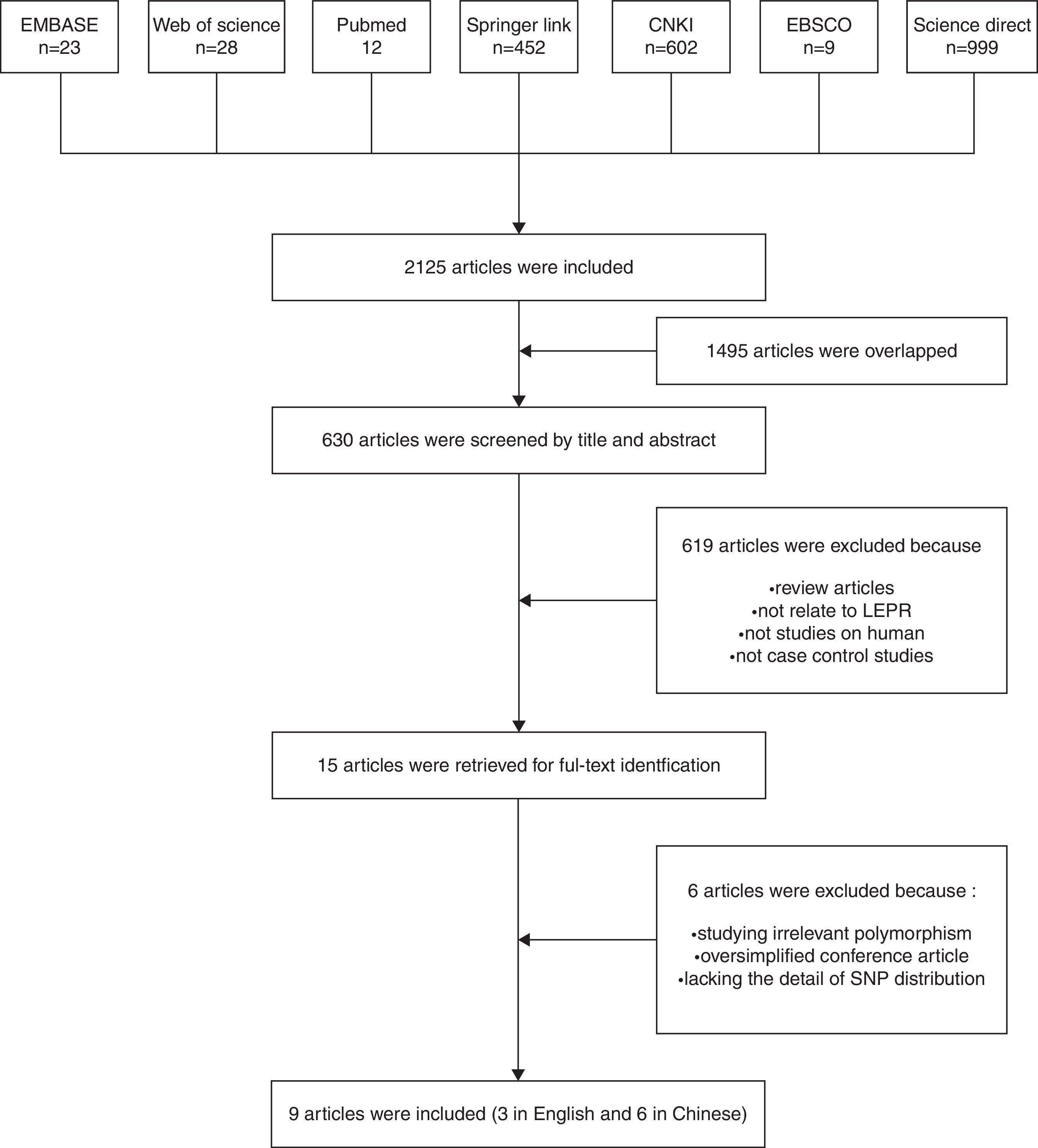
Materials and methodsLiterature reviewThe Excerpt Medica Database, Web of science, Pubmed, Springer Link, Chinese National Knowledge Infrastructure, EBSCO and Science Direct databases were searched to find the literatures that reported the relationship between the leptin receptor polymorphisms and risk for OSAS from December 1971 to October, 2014. The keywords used were ‘leptin receptor, obstructive sleep apnea, single nucleotide polymorphisms (SNPs)’, ‘leptin receptor, obstructive sleep apnea, polymorphism’, ‘leptin receptor, obstructive sleep apnea, allele’, ‘leptin receptor, OSAS, SNP’, ‘leptin receptor, OSAS, polymorphism’, ‘leptin receptor, OSAS, allele’, ‘lepr, obstructive sleep apnea, polymorphism’, ‘lepr, obstructive sleep apnea, allele’, ‘lepr, OSAS, SNP’, ‘lepr, OSAS, polymorphism’, ‘lepr, OSAS, allele’. Only the articles in English or Chinese with an English abstract were selected. After excluding duplicates, titles and abstracts were reviewed. The articles were included if they: (1) were case–control studies (compare the difference between patients and health controls); (2) had genotype polymorphisms in both cases and controls. The articles were excluded if they were: (1) review articles; (2) not related to leptin receptor; (3) animal or in vitro study; (4) not related to the relationship between OSAS and leptin receptor. The selection process is showed in Fig. 2.
This study was performed with the approval of the Ethical Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
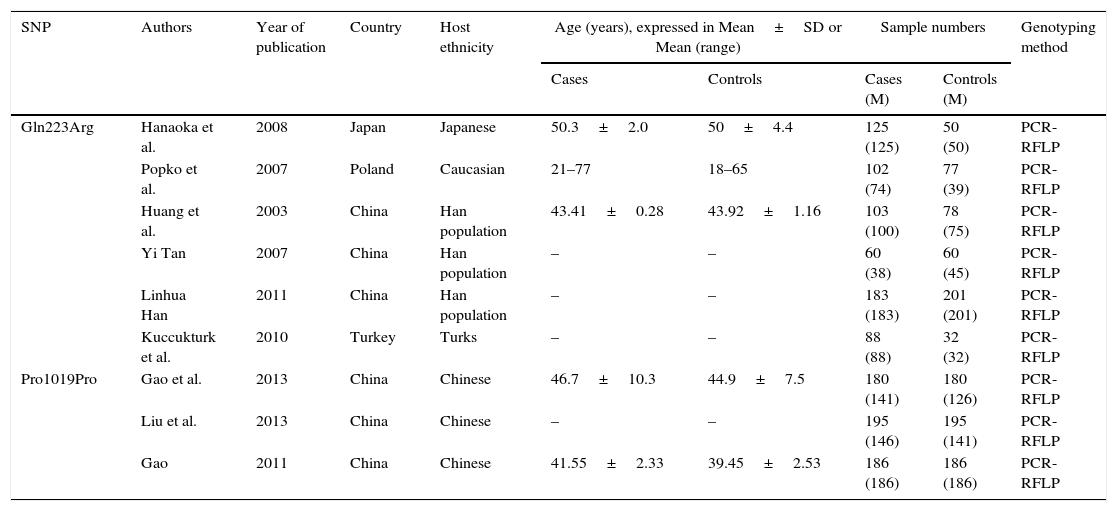
Data extractionFor each paper, information such as year of publication, name of the first author, etc. were extracted and summarized in Table 1.
Characteristics of the studies included in the analysis.
| SNP | Authors | Year of publication | Country | Host ethnicity | Age (years), expressed in Mean±SD or Mean (range) | Sample numbers | Genotyping method | ||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases (M) | Controls (M) | ||||||
| Gln223Arg | Hanaoka et al. | 2008 | Japan | Japanese | 50.3±2.0 | 50±4.4 | 125 (125) | 50 (50) | PCR-RFLP |
| Popko et al. | 2007 | Poland | Caucasian | 21–77 | 18–65 | 102 (74) | 77 (39) | PCR-RFLP | |
| Huang et al. | 2003 | China | Han population | 43.41±0.28 | 43.92±1.16 | 103 (100) | 78 (75) | PCR-RFLP | |
| Yi Tan | 2007 | China | Han population | – | – | 60 (38) | 60 (45) | PCR-RFLP | |
| Linhua Han | 2011 | China | Han population | – | – | 183 (183) | 201 (201) | PCR-RFLP | |
| Kuccukturk et al. | 2010 | Turkey | Turks | – | – | 88 (88) | 32 (32) | PCR-RFLP | |
| Pro1019Pro | Gao et al. | 2013 | China | Chinese | 46.7±10.3 | 44.9±7.5 | 180 (141) | 180 (126) | PCR-RFLP |
| Liu et al. | 2013 | China | Chinese | – | – | 195 (146) | 195 (141) | PCR-RFLP | |
| Gao | 2011 | China | Chinese | 41.55±2.33 | 39.45±2.53 | 186 (186) | 186 (186) | PCR-RFLP | |
SNP: single nucleotide polymorphism. M: male.
The fitness to the Hardy–Weinberg equilibrium (HWE) was tested by asymptotic Pearson's Chi-square test. The correlation between SNPs and OSAS risk was tested with odds ratios (OR) and the 95% confidence intervals (CIs). Q test and I2 test were used to test the heterogeneity between studies. The heterogeneity was considered significant if P<0.05. Fixed-effects model was considered when P>0.05; otherwise random-effects model was considered.12 The Begg's test and Egger's test were used to estimate the publication bias.13,14 The Stata 13.0 (College Station, TX) was used in Statistical analyses.
ResultsData extractionIn the literature search 2125 articles were extracted (Fig. 2). After removing the duplicates, 630 abstracts were examined. Fifteen original articles reporting the relationship between the human leptin receptor gene polymorphisms and OSAS risk were identified. Six articles were excluded after examining the full-text because they were irrelevant polymorphisms, oversimplified conference abstracts, or lacking the details of polymorphism distribution. Finally, 9 studies from 9 articles were included in this study.15–23 Three articles were published in English, while six articles were published in Chinese. Overall, five leptin receptor SNPs, including Lys109Arg, Lys656Asn, Pro1019Pro, G2458A, and Gln223Arg, were studied in these studies; however, only Gln233Arg and Pro1019Pro had multiple studies. The articles on other SNPs were no more than two; therefore, we chose Gln223Arg and Pro1019Pro as the target genes in our study.
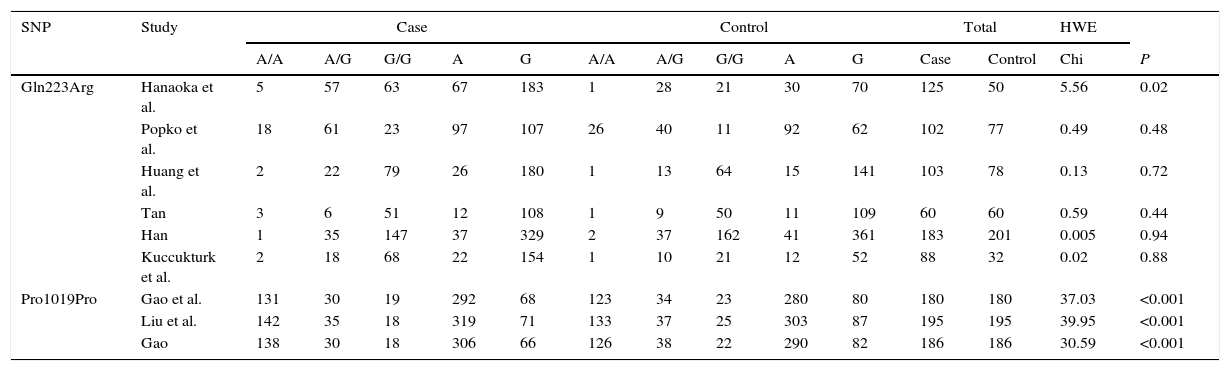
Characteristics of studiesThe races of study populations included Caucasian, Turks, Chinese and Japanese (Table 1). 1159 subjects (661 cases and 498 controls) were included in this study. The genotypes and allele distributions of SNPs are shown in Table 2. The genotype distributions in controls were deviated from HWE in 6 studies. Based on the information from CNBI SNPs database, the locations of each SNP are shown in Fig. 2.
Genotypes and allele distributions of SNPs in OSA patients and controls.
| SNP | Study | Case | Control | Total | HWE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/A | A/G | G/G | A | G | A/A | A/G | G/G | A | G | Case | Control | Chi | P | ||
| Gln223Arg | Hanaoka et al. | 5 | 57 | 63 | 67 | 183 | 1 | 28 | 21 | 30 | 70 | 125 | 50 | 5.56 | 0.02 |
| Popko et al. | 18 | 61 | 23 | 97 | 107 | 26 | 40 | 11 | 92 | 62 | 102 | 77 | 0.49 | 0.48 | |
| Huang et al. | 2 | 22 | 79 | 26 | 180 | 1 | 13 | 64 | 15 | 141 | 103 | 78 | 0.13 | 0.72 | |
| Tan | 3 | 6 | 51 | 12 | 108 | 1 | 9 | 50 | 11 | 109 | 60 | 60 | 0.59 | 0.44 | |
| Han | 1 | 35 | 147 | 37 | 329 | 2 | 37 | 162 | 41 | 361 | 183 | 201 | 0.005 | 0.94 | |
| Kuccukturk et al. | 2 | 18 | 68 | 22 | 154 | 1 | 10 | 21 | 12 | 52 | 88 | 32 | 0.02 | 0.88 | |
| Pro1019Pro | Gao et al. | 131 | 30 | 19 | 292 | 68 | 123 | 34 | 23 | 280 | 80 | 180 | 180 | 37.03 | <0.001 |
| Liu et al. | 142 | 35 | 18 | 319 | 71 | 133 | 37 | 25 | 303 | 87 | 195 | 195 | 39.95 | <0.001 | |
| Gao | 138 | 30 | 18 | 306 | 66 | 126 | 38 | 22 | 290 | 82 | 186 | 186 | 30.59 | <0.001 | |
SNPs: single nucleotide polymorphisms; OSA: Obstructive sleep apnea; HWE: Hardy–Weinberg equilibrium.
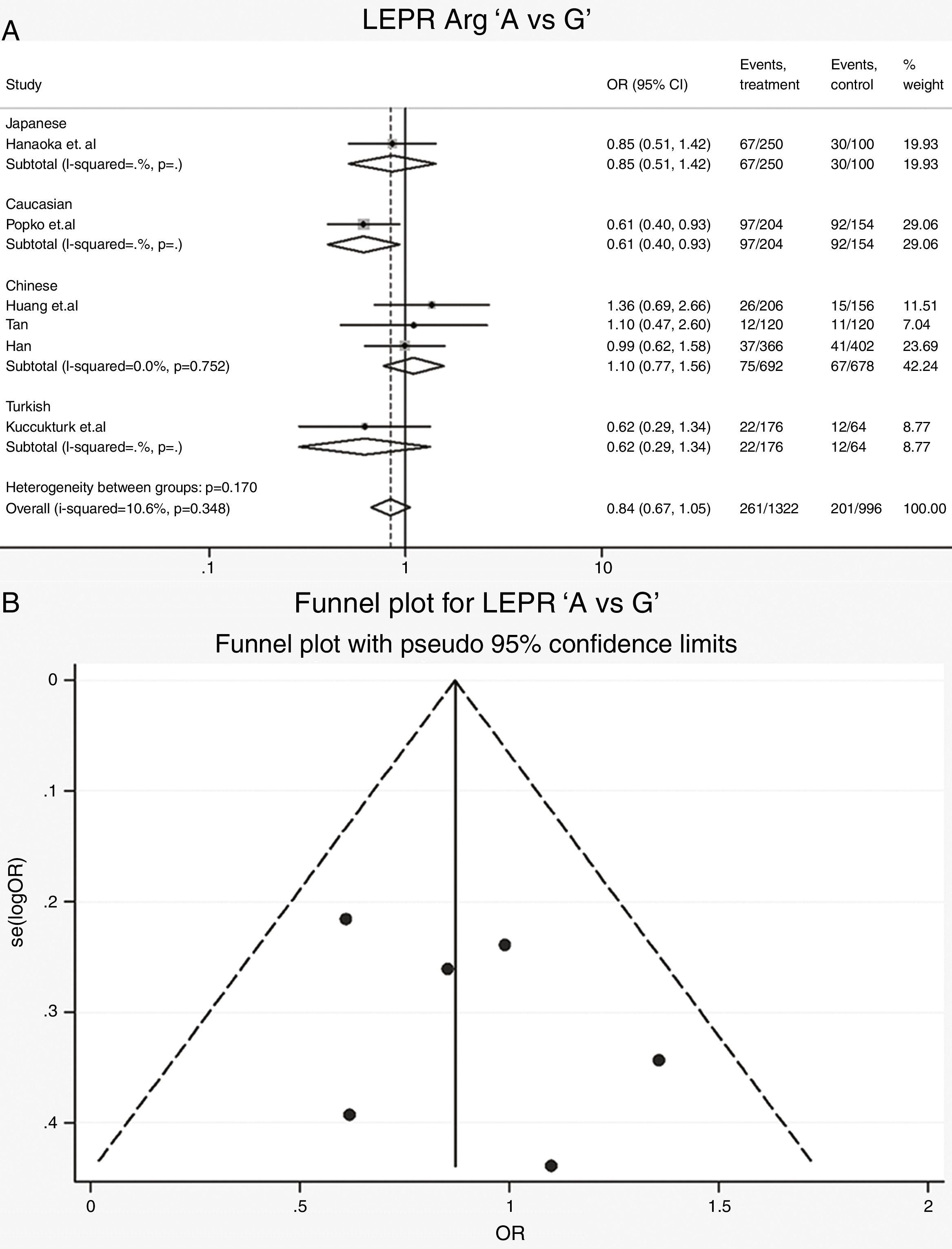
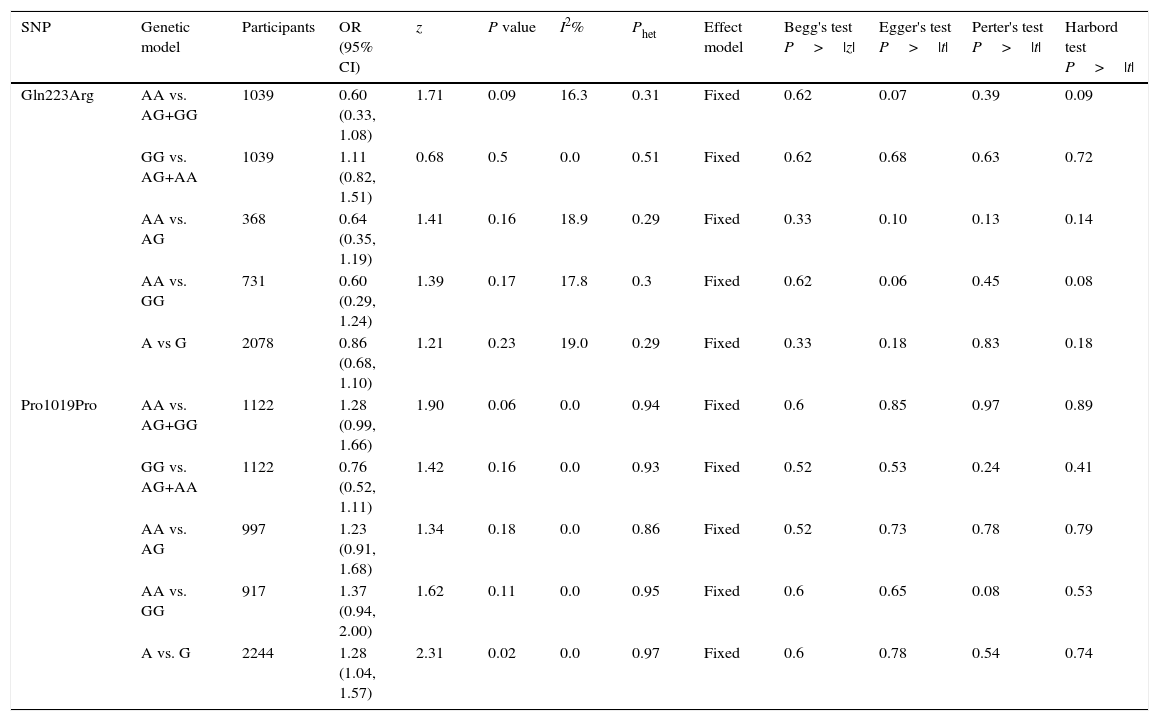
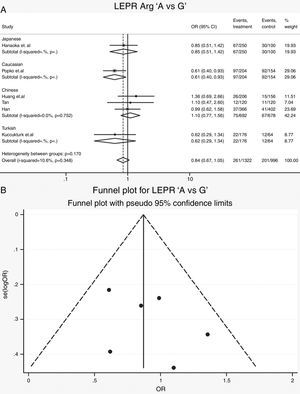
Leptin receptor Gln223Arg polymorphisms: Six case-control studies (661 cases and 498 controls) addressed the relationship between Gln223Arg polymorphisms and the risk of OSAS.16–21 The heterogeneity of the studies was not significant (Table 3, Phet=0.35, I2=10.6%). The OR (A vs. G alleles) using fixed-effects model was 0.84 (95% CIs: 0.67, 1.05), P=0.13. Other genetic models were also analyzed, however no correlation was identified (Table 3). In these genetic models the publication bias was not significant (Table 3). Allele comparison (A vs. G) between the ethnicities was performed (Fig. 3). Caucasians with G type had a higher risk of OSAS (P=0.01). The funnel plot is shown in Fig. 3.
Summary of comparative meta-analysis results.
| SNP | Genetic model | Participants | OR (95% CI) | z | P value | I2% | Phet | Effect model | Begg's test P>|z| | Egger's test P>|t| | Perter's test P>|t| | Harbord test P>|t| |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gln223Arg | AA vs. AG+GG | 1039 | 0.60 (0.33, 1.08) | 1.71 | 0.09 | 16.3 | 0.31 | Fixed | 0.62 | 0.07 | 0.39 | 0.09 |
| GG vs. AG+AA | 1039 | 1.11 (0.82, 1.51) | 0.68 | 0.5 | 0.0 | 0.51 | Fixed | 0.62 | 0.68 | 0.63 | 0.72 | |
| AA vs. AG | 368 | 0.64 (0.35, 1.19) | 1.41 | 0.16 | 18.9 | 0.29 | Fixed | 0.33 | 0.10 | 0.13 | 0.14 | |
| AA vs. GG | 731 | 0.60 (0.29, 1.24) | 1.39 | 0.17 | 17.8 | 0.3 | Fixed | 0.62 | 0.06 | 0.45 | 0.08 | |
| A vs G | 2078 | 0.86 (0.68, 1.10) | 1.21 | 0.23 | 19.0 | 0.29 | Fixed | 0.33 | 0.18 | 0.83 | 0.18 | |
| Pro1019Pro | AA vs. AG+GG | 1122 | 1.28 (0.99, 1.66) | 1.90 | 0.06 | 0.0 | 0.94 | Fixed | 0.6 | 0.85 | 0.97 | 0.89 |
| GG vs. AG+AA | 1122 | 0.76 (0.52, 1.11) | 1.42 | 0.16 | 0.0 | 0.93 | Fixed | 0.52 | 0.53 | 0.24 | 0.41 | |
| AA vs. AG | 997 | 1.23 (0.91, 1.68) | 1.34 | 0.18 | 0.0 | 0.86 | Fixed | 0.52 | 0.73 | 0.78 | 0.79 | |
| AA vs. GG | 917 | 1.37 (0.94, 2.00) | 1.62 | 0.11 | 0.0 | 0.95 | Fixed | 0.6 | 0.65 | 0.08 | 0.53 | |
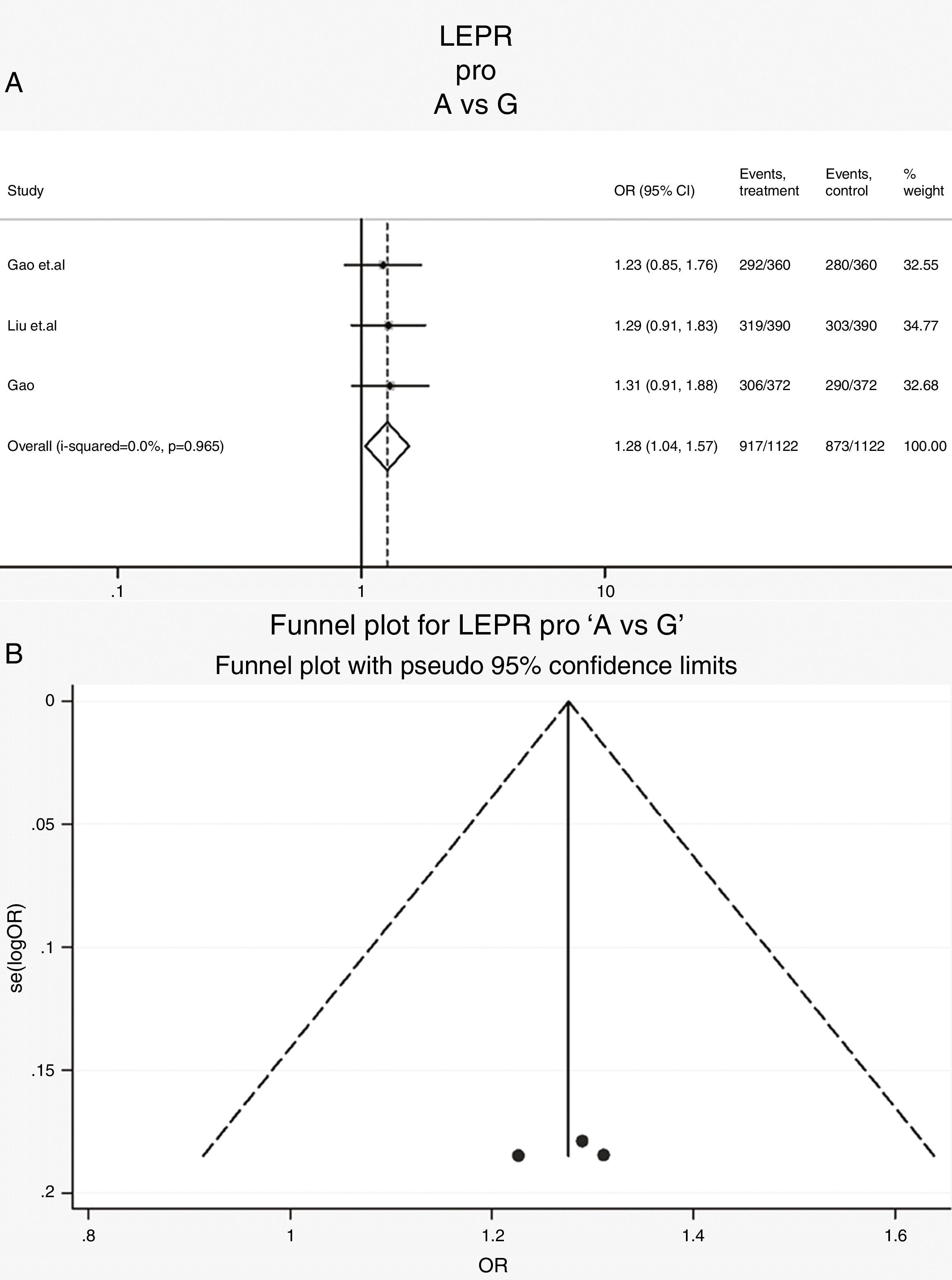
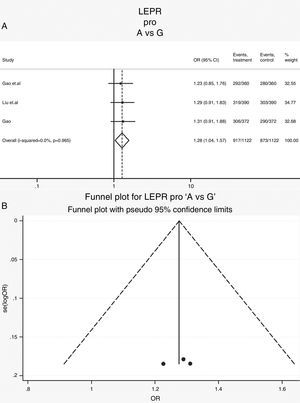
| A vs. G | 2244 | 1.28 (1.04, 1.57) | 2.31 | 0.02 | 0.0 | 0.97 | Fixed | 0.6 | 0.78 | 0.54 | 0.74 |
SNP: single nucleotide polymorphism; OR: odds ratio; CI: confidence interval.
Leptin receptor Pro1019Pro polymorphisms: Three case-control studies (561 cases and 561 controls) addressed the relationship between Pro1019Pro polymorphisms and the risk of OSAS.15,22,23 All the patients and controls were Chinese. The heterogeneity was not significant (Table 3, Phet=0.97, I2=0%). The OR (A vs. G alleles) using fixed-effects model was 1.28 (95% CIs: 1.04, 1.57), P=0.02. It suggests that the Chinese population with a type G had a higher risk of OSAS. Other genetic models were also analyzed, however no correlation was identified (Table 3). The Forrest plot in allele comparison (A vs. G) was performed by ethnicity (Fig. 4). The funnel plot is shown in Fig. 4.
DiscussionIn this study, we performed a meta-analysis to assess the correlation between leptin receptor Gln223Arg and Pro1019Pro polymorphisms and OSAS risk. The distributions of cases and controls were not indicated in the original data; however, the p value (0.234) and the numbers of patients and controls were mentioned in the original articles. We deduced the possible number by Fisher's exact test.24 The results are shown in Table 2. The analysis revealed no correlation between Gln223Arg polymorphisms and OSAS risk; whereas, Pro1019Pro polymorphisms are associated with OSAS risk in the Chinese population. In addition, five different models (Allele, Dominant, Heterozygote, Homozygote and Recessive) were performed in all SNPs. A subgroup analysis by ethnicities was also performed. Our results indicate that there is no significant correlation between Gln223Arg allele and OSAS risk. However, a significant correlation with OSAS risk in the Caucasian population, but not in the Chinese population, is observed.
We chose leptin receptor gene in our analysis because of its critical role in obesity, which is linked to OSAS. Phillips et al. have demonstrated that the leptin level in patients with OSAS is ∼1.5 times higher than controls (13.7/9.2ng/ml, P=0.02).25 Coutant et al. have shown a significant difference (23.8/3.8ng/ml).26 These results imply that the leptin level may be associated with OSAS risk. Animal model studies indicate that the loss-of-function mutation of leptin receptors cause leptin-resistant obesity in the mutant mice. It is reasonable to seek the mutations in leptin receptor genes, which lead to leptin-resistant obesity.
The rs number of Gln223Arg is rs1137101. It is a transition mutation (A>G). The transition changes the 223 amino acid residue Gln into Arg in receptor protein. It may change the three-dimensional conformation of this protein. Additionally, the SNP Gln223Arg is an extracellular domain that is the binding site of leptin. This mutation may change the binding ability of leptin receptors. On the other hand, the Pro1019Pro mutation does not change the protein conformation, but it is associated with OSAS risk in the Chinese population, which might be due to the influence of its linkage site. Further study such as multi-ethnic, linkage disequilibrium may reveal the function of Pro1019Pro.
Although the overall statistic in our study is non-significant, the correlation of Gln223Arg and OSAS risk varies by race. In the Chinese population, three genotypes are not associated with the risk of OSAS, while in the Polish population the G or Arg genotype is associated with a higher risk of OSAS (P=0.01). It implies that race may play an important role in the correlation between SNP and OSAS. On the other hand, although 5 case-control studies of Gln223Arg are included, only one study is related to the Caucasian population. Among the excluded articles, there were two conference abstracts involving Gln223Arg, but no details were included. Therefore, the results should be interpreted cautiously, and more studies should be included to confirm the correlation between the race and the risk of OSAS.
Apart from Gln223Arg, other SNPs may also be associated with the risk of OSAS. In the study of Hanaoka et al. wild-type alleles of the Gln223Arg and Lys656Asn SNPs have a significant resistant effect on mild OSAS (P=0.053 and 0.047), but have no correlation with the total risk of OSAS. It suggests that other SNPs of leptin receptors may influence the leptin function and/or the severity of OSAS.
ConclusionIn conclusion, this study has analyzed the published literature on the correlation between leptin receptor polymorphisms and OSAS risk. The results indicate that the Pro1019Arg polymorphisms, but not Gln223Arg polymorphisms, have a significant correlation with OSAS risk; however, the G allele polymorphism in Pro1019Pro and Arg allele polymorphism in Gln223Arg might be associated with a higher OSAS risk in the Chinese population or the Caucasian population, respectively.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Authors’ contributionSL and BX carried out the publication research, participated in data extraction and drafted the manuscript. BX, JL, and TL carried out the data analysis and participated in the design of the study. SL and BX conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
This study was supported by Scientific Research Foundation of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Grant No. 2014-004).