The influence of exercise on the pulmonary function is controverse, some studies have reported no sports influence, while the others have found positive correlation.
AimTo evaluate and compare the sports influence on pulmonary function: spirometry (VC, FVC, FEV1, FEV1/FVC), lung diffusing capacity (DLCO) and coefficient of the CO gas transfer (KCO) in two elite athletes groups and healthy sedentary controls.
MethodEqually divided into aerobic and anaerobic group, 60 elite athletes were recruited, as well as 43 age-matched, healthy sedentary controls. All of the participants performed basic anthropometric measurements, spirometry, DLCO and KCO at rest. Kruskal–Wallis one way ANOVA test was used to determine differences between groups; Mann–Whitney U test was used for inter-groups differences and Pearson coefficient for pulmonary variables and anthropometric parameters correlation. Statistical analyses were performed using the SPSS computer statistic program, version 20.
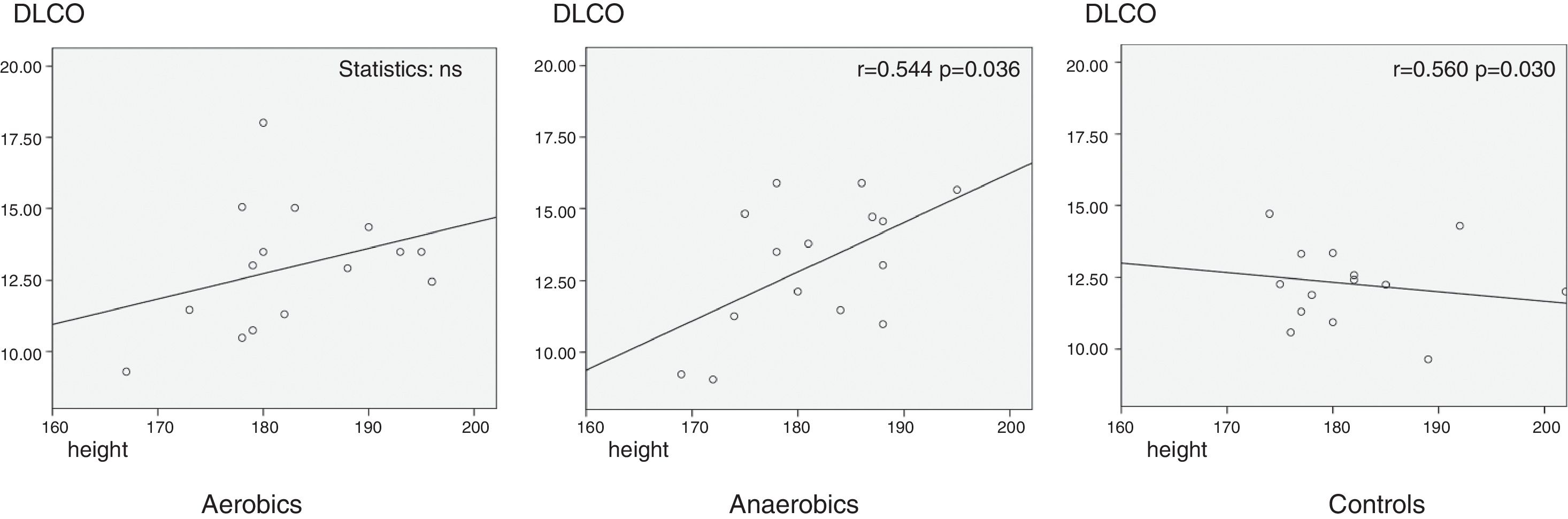
ResultsNo differences were found in pulmonary characteristics (spirometric function values, DLCO and KCO) in athletes and non-athletes at rest, as well as between aerobics and anaerobics. There were no correlations between the anthropometric parameters and the investigated respiratory function tests. DLCO (%) correlated positively with height in athletes playing anaerobic type of sport (karate and taekwondo) (p=0.036; r=0.544), and negatively in sedentary control group (p=0.030; r=−0.560). Regarding KCO, no differences were found.
ConclusionSpirometry indices and DLCO are not influenced either by aerobic or anaerobic training type, so benefits of sports on pulmonary indices or DLCO was not confirmed.
Single breath carbon monoxide diffusing capacity (DLCO) is one of the most valuable clinical tests for the pulmonary function assessment in clinical medicine and one of the most widely used tests for the pulmonary gas exchange, measuring the inspired and expired carbon monoxide partial pressure difference.1,2 It reflects alveolo-capillary membrane properties, particularly the movement of oxygen from the alveoli into the red blood cells, demonstrating the capillary gas uptake.1 In healthy individuals, DLCO is mainly predicted by height, gender and age.
Studies examining pulmonary function and DLCO in athletes are scarce and heterogeneous with some contradictory results. Some studies have shown no significant differences in the respiratory function between physically trained individuals and their age-matched sedentary controls, while others have found larger lung volumes in some particular types of sport, such as swimming.2
In order to add information about the influence of sport on DLCO, to define sport disciplines in terms of the health benefit, to document the effects of training on the pulmonary function and eventually add new follow-up measurements in sports, we have investigated the lung diffusing capacity in young elite athletes and their age-matched non-athletic, sedentary controls. Athletes were divided into two groups according to their different sport disciplines which cover two totally divergent ways of training: aerobic and anaerobic.
Materials and methodsParticipantsA total of 103 persons were investigated (all men, since there were no women in the examined professional sport disciplines). The sport group included 60 young elite athletes, divided into two groups according to the type of the sport involved: 30 elite football players (aerobics) and 30 karate and taekwondo players (anaerobics), aged (mean (SD)) 20.7 (4.1) and 21.9 (4.3) years, respectively. Control group consisted of 43 age-matched sedentary counterparts, aged 21.5 (3.4) years. Elite athlete was typically described as an ‘athlete who is systematically being trained for a minimum of five years and at least 15hours a week, and has been competing in international level tournaments’. Average training period for athletes was 12.4 (4.3) years with 19.1 (0.4) hours per week for football players and 11.6 (2.4) years and 19.1 (2.1) hours for karate and taekwondo players. Exclusion criteria were: past or current smoking, as well as any known chronic respiratory or cardiovascular disease. None of the athletes suffered from exercise-induced asthma, according to self-reports. Control group participants were not routinely engaged in any type of sport.
Ethical approvalThe research protocol was approved by Institutional Review Board for medical ethics and complied with the Declaration of Helsinki guidelines. All participants had given written informed consent before the inclusion.
Pulmonary dataAll experiments were performed using the same spirometer and analyzed with integrated software (MasterScreen Diffusion, Viasys HealthCare, Germany). The calibration of spirometar was checked on daily basis according to the American Thoracic Society/European Respiratory Society (ATS/ERS) criteria. All participants rested in sitting position for 10minutes before performing pulmonary function tests (PFT). First, the (relaxed) vital capacity (VC) was determined. After taking a series of normal breaths, the participants exhaled fully, following by a full inhalation. Several maneuvers of VC were performed and in some cases additional measurements were required, until the two highest VCs did not differ by more than 150ml. After determination of VC, the lung diffusing capacity for carbon monoxide (DLCO) was established as described before. A simulator test was performed daily, and the system was recalibrated before each individual test. During the test, the participants were asked to start with normal respiration, followed by a relaxed exhalation up to the residual volume and a quick inhalation to ≥90% VC from the gas source with 0.3% carbon monoxide. During the test participants were asked to exhale after 9–11s. The interval between trials was ≥4minutes. DLCO was presented unadjusted and as the percentage of the predicted value (% DLCO pred). DLCO per alveolar volume (VA) gives the coefficient of CO transfer (KCO), which was also presented as the predicted percentage KCO (KCO pred). After completion of DLCO measurements, participants were asked to perform maximal forced inspiratory and expiratory maneuvers after a short period of normal breathing to construct flow-volume curves. This procedure was repeated until the criteria set out by the ATS/ERS were met. We measured the following parameters: vital capacity-VC (measured in liters-L), forced vital capacity-FVC (L), forced expiratory volume in the first second-FEV1 (L), Peak Expiratory FlowPEF: (L/s) and FEV1/FVC ratio (%). The predicted values for FEV1 (FEV1pred), PEF (PEF pred) and DLCO were derived from reference value for European Community for Steel and Coal (ECSC).
Data analysisPulmonary parameters were presented by descriptive methods of statistics, as predicted and measured values, the later achieved both in liters and in percentages. Kruskal–Wallis one way ANOVA test was used to determine differences between groups with relation to the measured parameters; Mann–Whitney U test was used for inter-groups differences. Correlation between pulmonary variables and anthropometric parameters was determined by Pearson coefficient. Statistical analyses were performed by using the SPSS for Windows, version 20.
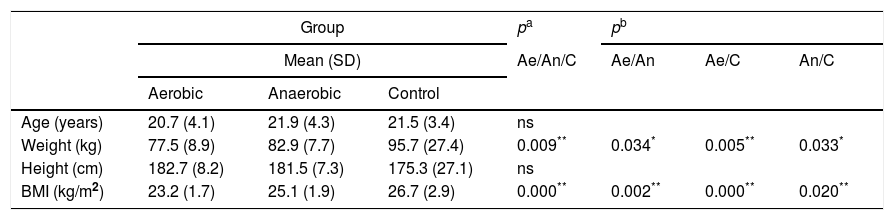
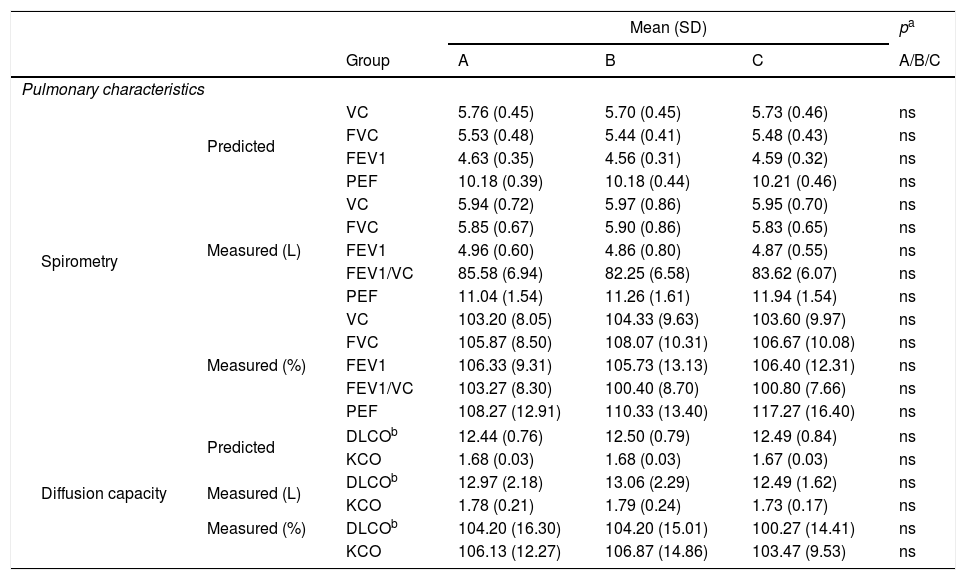
ResultsParticipant characteristics are shown in Table 1. No significant age-related differences were found. Significant differences were found in weight and BMI between aerobics and controls, aerobics and anaerobics, anaerobics and controls (p<0.05). The null hypothesis that the investigated parameters had the same distribution across groups was tested. No difference was found regarding predicted and measured VC, FVC, FEV1 and PEF values, as well as with regard to VC, FVC, FEV1, FEV1/VC and PEF measured values (both in liters and in percentages) among aerobics, non-aerobics and controls. Also, no difference was found in DLCO and KCO in aerobics, non-aerobics and controls (Table 2).
Patient characteristics.
| Group | pa | pb | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Ae/An/C | Ae/An | Ae/C | An/C | |||
| Aerobic | Anaerobic | Control | |||||
| Age (years) | 20.7 (4.1) | 21.9 (4.3) | 21.5 (3.4) | ns | |||
| Weight (kg) | 77.5 (8.9) | 82.9 (7.7) | 95.7 (27.4) | 0.009** | 0.034* | 0.005** | 0.033* |
| Height (cm) | 182.7 (8.2) | 181.5 (7.3) | 175.3 (27.1) | ns | |||
| BMI (kg/m2) | 23.2 (1.7) | 25.1 (1.9) | 26.7 (2.9) | 0.000** | 0.002** | 0.000** | 0.020** |
Pulmonary characteristics (spirometrics, diffusion capacity) for aerobics (A), non-aerobics (B) and controls (C).
| Mean (SD) | pa | |||||
|---|---|---|---|---|---|---|
| Group | A | B | C | A/B/C | ||
| Pulmonary characteristics | ||||||
| Spirometry | Predicted | VC | 5.76 (0.45) | 5.70 (0.45) | 5.73 (0.46) | ns |
| FVC | 5.53 (0.48) | 5.44 (0.41) | 5.48 (0.43) | ns | ||
| FEV1 | 4.63 (0.35) | 4.56 (0.31) | 4.59 (0.32) | ns | ||
| PEF | 10.18 (0.39) | 10.18 (0.44) | 10.21 (0.46) | ns | ||
| Measured (L) | VC | 5.94 (0.72) | 5.97 (0.86) | 5.95 (0.70) | ns | |
| FVC | 5.85 (0.67) | 5.90 (0.86) | 5.83 (0.65) | ns | ||
| FEV1 | 4.96 (0.60) | 4.86 (0.80) | 4.87 (0.55) | ns | ||
| FEV1/VC | 85.58 (6.94) | 82.25 (6.58) | 83.62 (6.07) | ns | ||
| PEF | 11.04 (1.54) | 11.26 (1.61) | 11.94 (1.54) | ns | ||
| Measured (%) | VC | 103.20 (8.05) | 104.33 (9.63) | 103.60 (9.97) | ns | |
| FVC | 105.87 (8.50) | 108.07 (10.31) | 106.67 (10.08) | ns | ||
| FEV1 | 106.33 (9.31) | 105.73 (13.13) | 106.40 (12.31) | ns | ||
| FEV1/VC | 103.27 (8.30) | 100.40 (8.70) | 100.80 (7.66) | ns | ||
| PEF | 108.27 (12.91) | 110.33 (13.40) | 117.27 (16.40) | ns | ||
| Diffusion capacity | Predicted | DLCOb | 12.44 (0.76) | 12.50 (0.79) | 12.49 (0.84) | ns |
| KCO | 1.68 (0.03) | 1.68 (0.03) | 1.67 (0.03) | ns | ||
| Measured (L) | DLCOb | 12.97 (2.18) | 13.06 (2.29) | 12.49 (1.62) | ns | |
| KCO | 1.78 (0.21) | 1.79 (0.24) | 1.73 (0.17) | ns | ||
| Measured (%) | DLCOb | 104.20 (16.30) | 104.20 (15.01) | 100.27 (14.41) | ns | |
| KCO | 106.13 (12.27) | 106.87 (14.86) | 103.47 (9.53) | ns | ||
VC, vital capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in1 second; PEF, peak expiratory flow; VC, Tiffeneau-Pinelli index; DLCO, diffusion capacity for carbon monoxide; KCO, transfer coefficient for carbon monoxide.
Investigating anthropometric influence on respiratory function, the only significant correlations were found between DLCO (%) and height, which might be due to different alveolar volumes, found to be positive in anaerobic group (p=0.036; r=0.544), and negative in the control group (p=0.030; r=−0.560) (Graph 1).
DiscussionThe present study has shown no difference in lung volumes, lung diffusing capacity and gas transfer coefficient between aerobic athletes, anaerobic athletes and their age-matched controls at rest. Our investigation has shown no influence of age, body weight, body height and BMI on lung volumes, as previously reported.2 Strong positive correlation was found between height and DLCO in athletes playing anaerobic type of sport (karate) (the higher the athlete is, the higher DLCO he has) and negative correlation in controls (the taller the person is, the lower DLCO is). We did not observe correlations between DLCO/VA ratio and height.
According to some studies, athletes have higher lung volumes than sedentary controls, mostly caused by respiratory adaptations to training.3–5 However, published material about training influence on DLCO enhancement is limited. In 1967, several authors have reported higher DLCO in athletes (specially swimmers, when compared to sedentary controls), suggesting that increased DLCO was due to physical training under the continuous oxygen delivery circumstances.6
On the other hand, some studies have also denied the influence of training on athlete's DLCO: a vigorous five-months endurance training has shown lowering of the heart rate, but no DLCO changes.7 In elite swimmers, enhanced DLCO could be caused by larger lung volumes, not by DLco/VA ratio increase. It could be explained by the specific way of training, which might be the reason for DLCO increase. It should be borne in mind that higher DLCO in athletes could also be caused by the self-selection bias. A very recent study has shown that endurance-trained athletes appear to have differences within the pulmonary membrane that facilitate the increased O2 demand for the high-level exercise, as it was noticed that athletes had a greater DLCO and greater DM at 80 and 90% of VO2 max compared to non-athletes.8
However, it was suggested that there should be an upper limit for the pulmonary expansion at which DLCO reaches its maximum. The apparent oxygen diffusing capacity (DLO2) approached a plateau or upper limit as the work load increases, by reaching its submaximal levels.9,10 Repeated, high intensity exercise may reduce the integrity of the lungs over the years – leading to DLCO diminishment, which is increased with age and particularly and almost immediately after exercise.7 Also, the likelihood of developing higher arterial desaturation in trained men is less during submaximal exercise when compared to the untrained, because of the lung diffusion capacity limitation and the fact that mechanical constraints of respiration occur at maximal exercise, or close to it.7,8
Our study has shown no differences in pulmonary indices between aerobics and anaerobis at rest. However, some other studies have shown different lung volumes between athletes and non-athletes.3 Considering the type of the sport examined, karate, we did not expect huge respiratory system changes, concordant with earlier studies.4
The results of the present study suggest that different sport disciplines have no influence on the pulmonary function indices, which consequently cannot be used for monitoring the sport achievements.
Study limitations: controls were significantly overweight compared with the other two groups, which might have impact on DLCO. Results of the present study are limited to male subjects only (no elite female athletes were available in the disciplines we looked at). The sample size was limited by the number of national representatives in Serbia engaged in specific sport disciplines. As the study was performed at the beginning of the training session, we were not able to test whether the lung diffusing capacity would be subject to change after a pause in the intensive training session.
Conflicts of interestThe authors have no conflicts of interest to declare.