Asbestos is still the leading cause of occupational cancer mortality worldwide. Asbestos-related lung cancer (LC) and malignant pleural mesothelioma (MPM) prognosis is still poor especially at advanced stage, so early diagnosis biomarkers are needed. MicroRNAs (miRNAs) have been proposed as potential early diagnostic biomarkers of asbestos-related LC and MPM.
AimTo evaluate the role of miRNAs as diagnostic and prognostic biomarkers of asbestos-related LC and MPM by performing a literature systematic review and meta-analysis.
MethodsMEDLINE, EMBASE via Ovid, PUBMED and Cochrane library databases were systematically searched up to April 2023 to identify relevant articles. A grey literature search was also conducted using the Google Scholar platform. MeSH and free text terms for ‘asbestos’, ‘occupational exposure’, ‘lung cancer’, ‘mesothelioma’ and ‘miRNAs’ were used to search the literature. Our systematic review protocol was registered in the PROSPERO database. Study quality was assessed via the Newcastle-Ottawa Scale.
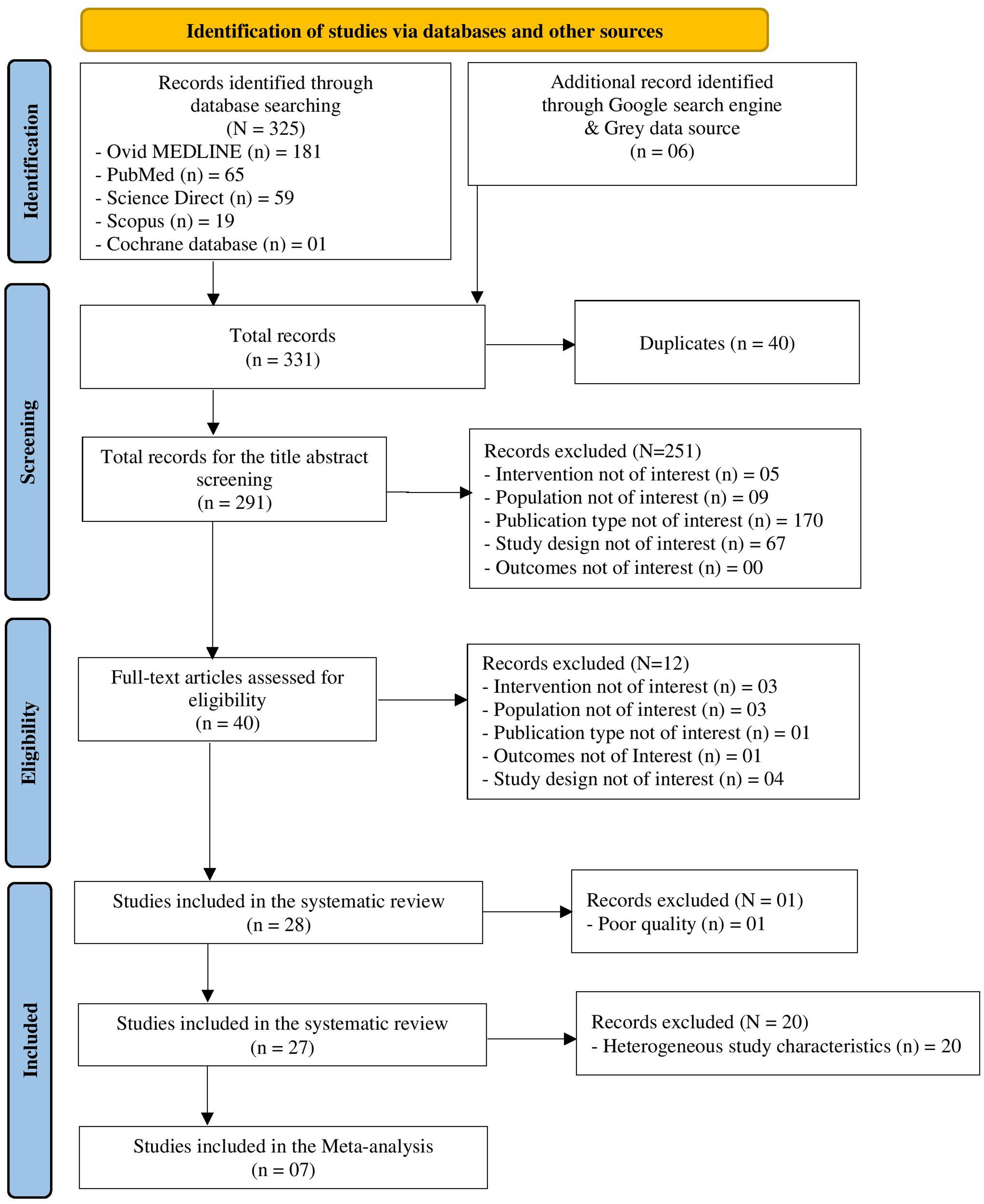
ResultsFrom the search, 331 articles were retrieved, and, after applying our selection criteria, and exclusion of one study for poor quality, 27 studies were included in the review. Most of the studies were hospital-based case-control, conducted in Europe, and evaluated MPM among men only. MiRNAs expression was measured mainly in plasma or serum. MiR-126, miR-132–3p, and miR-103a-3p were the most promising diagnostic biomarkers for MPM, and we estimated a pooled area under the curve (AUC) of 85 %, 73 %, and 50 %, respectively. In relation to MPM prognosis, miR-197‑3p resulted associated with increased survival time. MiR-126, alone and combined with miR-222, was confirmed associated also to LC diagnosis, together with miR-1254 and miR-574–5p; no miRNA was found associated to LC prognosis.
ConclusionBased on our systematic literature review there is suggestive evidence that the expression of specific miRNAs in the blood serum or plasma are associated with asbestos-related LC and MPM diagnosis and prognosis. Further large longitudinal studies are urgently needed to validate these findings and elucidate the underlying mechanisms given the potential important implications for patients’ survival.
Globally, it has been estimated that asbestos is still the leading cause of morbidity, disability, and mortality for occupational cancer accounting for 4120 (3060–5240) thousand DALYs (Disability-Adjusted Life Years) and 236 (176–296) thousand deaths. In relation to the specific cancer type, lung cancer (LC) is the most frequent, with 199 (140–257) thousand deaths, and malignant pleural mesothelioma (MPM) is the rarest, with 26.8 (24.3–28.6) thousand deaths, but it is virtually only caused by asbestos.1 The International Agency for Research on Cancer (IARC) has classified asbestos as a known human carcinogen not only for LC and MPM, but also for all mesothelioma types, larynx, and ovarian cancer, and, with weaker evidence, for throat, stomach, and colorectum malignancies.2 Moreover, chronic degenerative pleural and interstitial lung diseases, such as asbestosis are caused by asbestos exposure,3 so increasing the associated global morbidity and mortality burden.4 Of note, asbestos exposure is not only occupational, but it may occur in the home, and in surroundings of contaminated worksites with potential exposure of the most vulnerable, such as children and pregnant women.5,6 Regrettably, only 69 of the world's 195 countries have banned asbestos.7 The World Health Organization (WHO) estimated that 125 million people worldwide are still exposed to asbestos, and considering the long cancer latency (up to 60 years for MPM) the expected associated cancer burden won't decrease in the near future.8 Despite important advances in cancer therapy, both LC and MPM have poor prognosis, especially if diagnosed at a late stage. In addition, there is no agreed standard treatment for MPM whose median survival is less than one year from diagnosis.9 Cancer screening programs among ex-exposed to asbestos using low-dose chest CT-scans have been proposed,10 but so far none has started yet due to uncertain cost-benefits and challenges in risk stratification to identify which subgroup of subjects would benefit the most. Therefore, non-invasive biomarkers for risk stratification, and earlier cancer detection are urgently needed to improve overall survival and quality of life, as was recently recommended by the European MPM guidelines.11 MicroRNAs (miRNAs) are short, endogenous, non-coding ribonucleic acids that have been suggested as potential candidates. MiRNAs regulate key processes in cells and signaling pathways involved in lung tumorigenesis, such as cell proliferation, differentiation, angiogenesis, apoptosis, invasion, and metastasis by regulating gene expression at the post-transcriptional level,12 and are potential molecular targets for cancer therapies. Also, the availability of miRNAs in several accessible biological fluids and exhaled breath condensate (EBC) make them ideal candidates for liquid biopsies. Changes in miRNAs expression have been associated with diagnosis and prognosis of several chronic diseases and cancers,13 but the evidence for asbestos-related LC and MPM is still scarce and inconsistent. Therefore, the aim of our systematic literature review is to evaluate the role of miRNAs as diagnostic and prognostic biomarkers of asbestos-related LC and MPM.
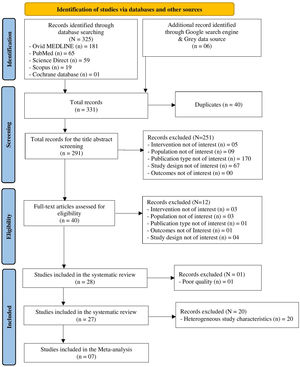
MethodsWe performed the systematic review according to the Cochrane Handbook for Systematic Reviews14 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.15 MeSH and free text terms for ‘asbestos’, ‘occupational exposure’, ‘lung cancer’, ‘mesothelioma’ and ‘miRNAs’ were used to search the literature in electronic databases: Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE) via Ovid platform, PubMed, and Cochrane Library (search period: January 1990 to April 2023) (see detailed search strategy in supplementary Table 1s). A grey literature search was conducted using the Google search engine platform to identify relevant studies not captured through database searches. Our systematic review protocol is registered in the PROSPERO database (registration Number: CRD42023414412 accessible at crd.york.ac.uk/prospero/display_record.php?ID=CRD42023414412). The studies retrieved from our electronic search were reviewed following the Population (P), Interventions (I), Comparators (C), Outcomes (O), Study Design (S), and Time Frame (T) model and screened for suitability according to our inclusion criteria (see PICOS-T criteria in supplementary Table 2s) by two reviewers independently (DM and SDM). A third reviewer was available in case of any disagreement (PC). Relevant data from each included study were extracted into an ad hoc Microsoft Excel (Microsoft Corp., Redmond, WA) table. Each study was appraised for quality using the Newcastle-Ottawa Scale (NOS) for observational studies.15 Studies of very poor quality were not included. For the miRNAs reported at least by two similar studies in association with diagnosis and/or prognosis of asbestos-related LC and/or MPM, a meta-analysis was performed to estimate a pooled quantitative diagnostic and/or prognostic accuracy using the AUC (Area Under the Curve) and its 95 % Confidence Intervals (CIs). We extracted the AUCs and their standard errors (s.e.) from the studies. We displayed graphically in a forest plot the pooled AUCs and their asymptotic intervals (AUC +/- 1.96*s.e.) on a non-transformed scale. Random effect methods16 were applied in case of high heterogeneity (I2>50 %). A potential small study effect bias was evaluated using Egger test and visualised using a funnel plot. The STATA v.17 (Stata Corp LP, College Station, TX) software was used for all analyses.
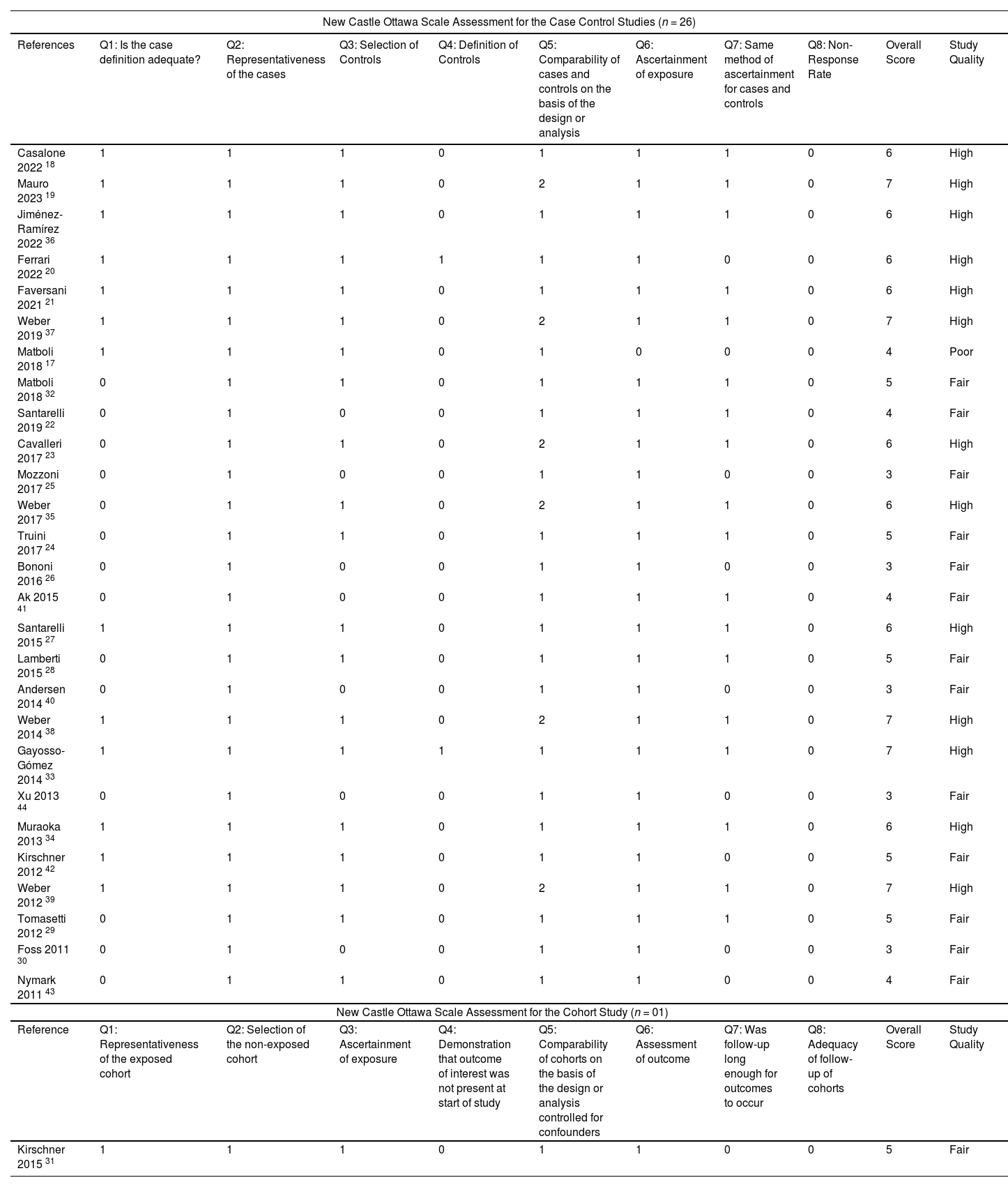
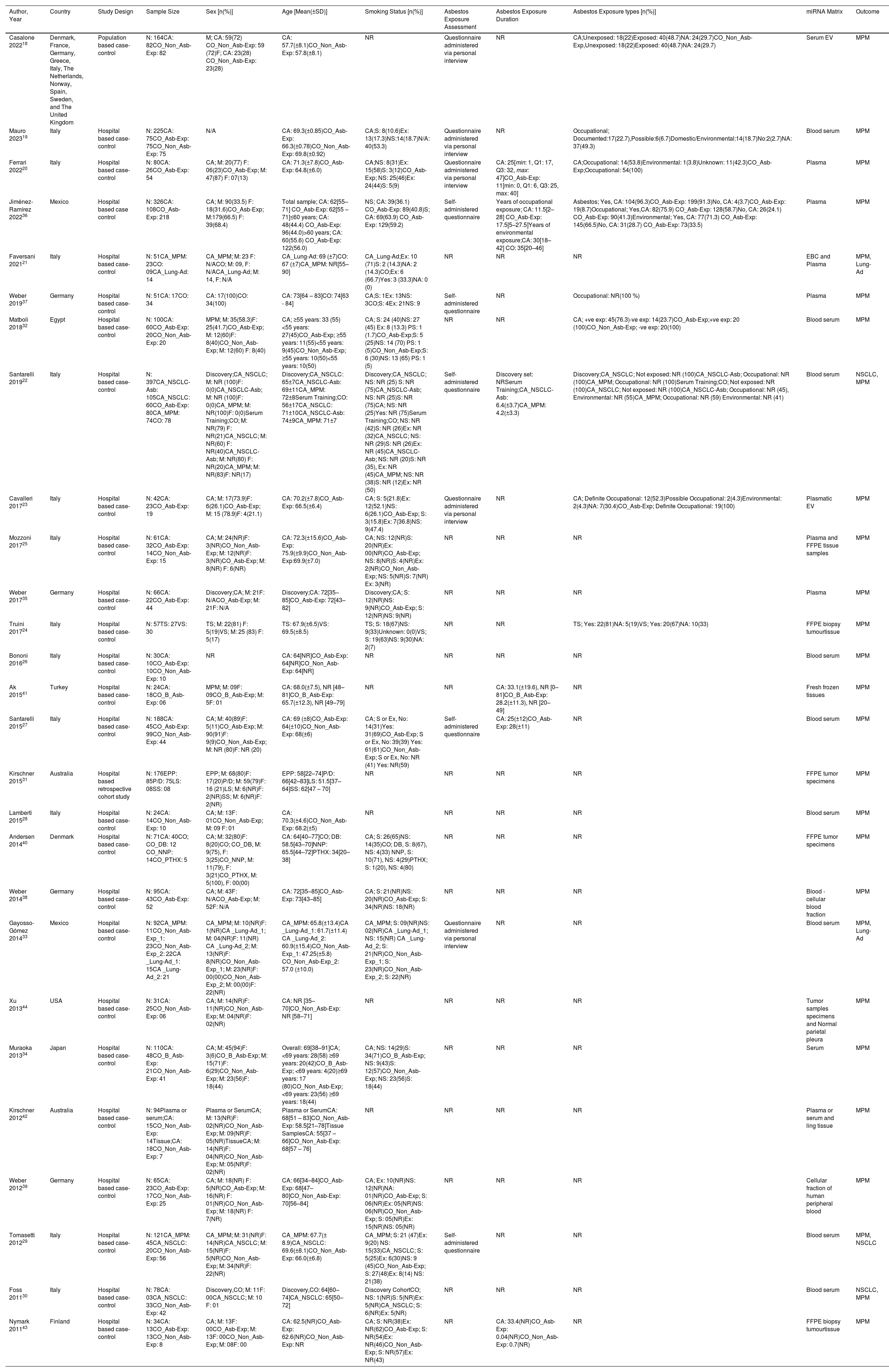
ResultsThe literature search retrieved a total of 331 citations. After duplicate removal and application of our selection criteria, 28 articles were included in the review. The quality of the included studies was scored between fair (n = 14), and high (n = 13); only one study17 was removed due to poor quality (see the New-Castle Ottawa quality of studies assessment scale in Table 1). Among the 27 studies included in the final review, only seven were selected as suitable for the meta-analysis (see PRISMA flow chart14 in Fig. 1). The characteristics of the 27 included studies are summarized in Table 2. Most studies were conducted in Europe, specifically in Italy (n = 13).18-30 Regarding the study design, all studies were hospital-based case-controls, but one study31 was a hospital-based retrospective cohort study. Most studies (n = 18) had low sample size. MiRNAs expression was evaluated mostly in blood serum or plasma19,20,22,26-30,32-37 and serum or plasma extracellular vesicles (EV).18,23,38,39 Some studies evaluated miRNA expression directly in the lung tumour tissue,23-25,31,40-42 and one in the EBC.21 The most frequently evaluated disease outcome was MPM, followed by non-small cell lung cancer (NSCLC)22,29,30 and lung adenocarcinoma.21,33 In most studies (n = 15), asbestos-exposed cancer-free hospital patients were used as controls. All but two studies19,26 were only among men. The age range was 52 – 73 years for MPM cases, 61 – 69 for LC cases, 58 – 74 years for non-asbestos-exposed controls and 55 – 76 for asbestos-exposed controls. Ten studies18-20,22,23,27,29,33,36,37 reported the asbestos exposure assessment method, either via personal interview or self-administered questionnaire and six studies20,22,27,36,41,43 calculated the asbestos exposure duration in years. The most frequent type of asbestos exposure was occupational. The majority of studies reported smoking status and some studies19,20,22-25,29,30,32-40 managed to include never smokers only; however seven studies18,26,28,31,41,42,44 did not report the smoking status. All included studies reported miRNAs diagnostic accuracy, and two studies reported the prognostic accuracy19,31 (Table 3). The miRNAs most frequently reported in association with MPM diagnosis were miR-126,22,25,27,29,40 miR-103a-3p,20,36-38 and miR-132–3p.35,37 Of note, miR-126 was confirmed alone,29 and combined with miR-222 22 also for LC diagnosis. Other miRNAs resulted associated with LC, but only by single studies, were miR-1254 and miR-574–5p,30 and let-7f-5p, miR-518f-3p, miR-597–5p, miR-1260a.21 In relation to types of miRNAs perturbations, several miRNAs associated with MPM diagnosis resulted up-regulated,18,19,22,26,30,32-34,41,42 others down-regulated.20,23-25,27,29,31,35-37,39 For LC, all miRNAs resulted up-regulated,21,30 but let-7f-5p.21 Most studies (n = 16) 18,20-24,26,31-33,37,40-44 estimated miRNA expression difference between cases and controls as fold change (FC) using different cut-off thresholds. Some studies managed to adjust tests’ p-values for multiple testing for MPM diagnosis 18,19,23,30,31,40,41,44 and LC diagnosis.21 Eight studies.19,25,32,35-37,39,43 matched cases and controls by potential confounders (e.g. age, sex, smoking status, or asbestos exposure) while nine studies 18,20-24,27,31,40 controlled for them in the statistical analysis. Ten studies 20,21,25,28,30,32-34,40,41 reported also cancer stages and the most frequent was stage I. Twenty-two studies 19-25,27,28,30,31,33-38,40-44 reported the histological subtypes; the most frequent type for asbestos-related LC was adenocarcinoma and for MPM was epithelioid. In relation to diagnostic accuracy (Table 4), the miRNAs with the highest values for MPM were: miR-103a-3p 38 with 86 % sensitivity, 63 % specificity and AUC of 0.76; miR-126 29 with 80 % sensitivity, 60 % specificity and AUC of 0.75(0.62–0.89) and miR-132–3p 35 with 86 % sensitivity, 61 % specificity and AUC of 0.91(0.8–1.0). In relation to MPM prognosis, miR-197‑3p 19 was associated with an increased survival for epithelioid type of 13.5 (±0.6) months, for sarcomatoid of 7.9 (±0.7) months, and for biphasic of 12.4 (±0.6) months. Also, six miRNAs combined (miR-21–5p, miR-23a-3p, miR-30e-5p, miR-221–3p, miR-222–3p, miR-31–5p) 31 resulted associated to 57.2 (45.83–90.48) months to 6.4 (1.94–8.28) months increased survival. In relation to asbestos-related LC diagnosis, miR-126 was confirmed associated also to LC diagnosis, alone,29 and in combination with miR-222.22 Also, miR-1254 and miR-574–5p30 were found associated with early-stage NSCLC even months before clinical diagnosis. No miRNAs were reported in association to survival for asbestos-related LC.
Quality assessment of the included studies via Newcastle Ottawa Scale.
| New Castle Ottawa Scale Assessment for the Case Control Studies (n = 26) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| References | Q1: Is the case definition adequate? | Q2: Representativeness of the cases | Q3: Selection of Controls | Q4: Definition of Controls | Q5: Comparability of cases and controls on the basis of the design or analysis | Q6: Ascertainment of exposure | Q7: Same method of ascertainment for cases and controls | Q8: Non-Response Rate | Overall Score | Study Quality |
| Casalone 2022 18 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | High |
| Mauro 2023 19 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | High |
| Jiménez-Ramírez 2022 36 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | High |
| Ferrari 2022 20 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 | High |
| Faversani 2021 21 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | High |
| Weber 2019 37 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | High |
| Matboli 2018 17 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 4 | Poor |
| Matboli 2018 32 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | Fair |
| Santarelli 2019 22 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 4 | Fair |
| Cavalleri 2017 23 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 6 | High |
| Mozzoni 2017 25 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | Fair |
| Weber 2017 35 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 6 | High |
| Truini 2017 24 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | Fair |
| Bononi 2016 26 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | Fair |
| Ak 2015 41 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 4 | Fair |
| Santarelli 2015 27 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | High |
| Lamberti 2015 28 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | Fair |
| Andersen 2014 40 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | Fair |
| Weber 2014 38 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | High |
| Gayosso-Gómez 2014 33 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | High |
| Xu 2013 44 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | Fair |
| Muraoka 2013 34 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | High |
| Kirschner 2012 42 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 | Fair |
| Weber 2012 39 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | High |
| Tomasetti 2012 29 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | Fair |
| Foss 2011 30 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | Fair |
| Nymark 2011 43 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 4 | Fair |
| New Castle Ottawa Scale Assessment for the Cohort Study (n = 01) | ||||||||||
| Reference | Q1: Representativeness of the exposed cohort | Q2: Selection of the non-exposed cohort | Q3: Ascertainment of exposure | Q4: Demonstration that outcome of interest was not present at start of study | Q5: Comparability of cohorts on the basis of the design or analysis controlled for confounders | Q6: Assessment of outcome | Q7: Was follow-up long enough for outcomes to occur | Q8: Adequacy of follow-up of cohorts | Overall Score | Study Quality |
| Kirschner 2015 31 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 5 | Fair |
Characteristics of the 27 studies included in the systematic review.
| Author, Year | Country | Study Design | Sample Size | Sex [n(%)] | Age [Mean(±SD)] | Smoking Status [n(%)] | Asbestos Exposure Assessment | Asbestos Exposure Duration | Asbestos Exposure types [n(%)] | miRNA Matrix | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Casalone 202218 | Denmark, France, Germany, Greece, Italy, The Netherlands, Norway, Spain, Sweden, and The United Kingdom | Population based case-control | N: 164CA: 82CO_Non_Asb-Exp: 82 | M; CA: 59(72) CO_Non_Asb-Exp: 59 (72)F; CA: 23(28) CO_Non_Asb-Exp: 23(28) | CA: 57.7(±8.1)CO_Non_Asb-Exp: 57.8(±8.1) | NR | Questionnaire administered via personal interview | NR | CA;Unexposed: 18(22)Exposed: 40(48.7)NA: 24(29.7)CO_Non_Asb-Exp,Unexposed: 18(22)Exposed: 40(48.7)NA: 24(29.7) | Serum EV | MPM |
| Mauro 202319 | Italy | Hospital based case-control | N: 225CA: 75CO_Asb-Exp: 75CO_Non_Asb-Exp: 75 | N/A | CA: 69.3(±0.85)CO_Asb-Exp: 66.3(±0.78)CO_Non_Asb-Exp: 69.8(±0.92) | CA;S: 8(10.6)Ex: 13(17.3)NS:14(18.7)N/A: 40(53.3) | Questionnaire administered via personal interview | NR | Occupational; Documented:17(22.7),Possible:6(6.7)Domestic/Environmental:14(18.7)No:2(2.7)NA: 37(49.3) | Blood serum | MPM |
| Ferrari 202220 | Italy | Hospital based case-control | N: 80CA: 26CO_Asb-Exp: 54 | CA; M: 20(77) F: 06(23)CO_Asb-Exp; M: 47(87) F: 07(13) | CA: 71.3(±7.8)CO_Asb-Exp: 64.8(±6.0) | CA;NS: 8(31)Ex: 15(58)S: 3(12)CO_Asb-Exp; NS: 25(46)Ex: 24(44)S: 5(9) | Questionnaire administered via personal interview | CA: 25[min: 1, Q1: 17, Q3: 32, max: 47]CO_Asb-Exp: 11[min: 0, Q1: 6, Q3: 25, max: 40] | CA;Occupational: 14(53.8)Environmental: 1(3.8)Unknown: 11(42.3)CO_Asb-Exp;Occupational: 54(100) | Plasma | MPM |
| Jiménez-Ramírez 202236 | Mexico | Hospital based case control | N: 326CA: 108CO_Asb-Exp: 218 | CA; M: 90(33.5) F: 18(31.6)CO_Asb-Exp; M:179(66.5) F: 39(68.4) | Total sample; CA: 62[55–71] CO_Asb-Exp: 62[55 – 71]≤60 years; CA: 48(44.4) CO_Asb-Exp: 96(44.0)>60 years; CA: 60(55.6) CO_Asb-Exp: 122(56.0) | NS; CA: 39(36.1) CO_Asb-Exp: 89(40.8)S; CA: 69(63.9) CO_Asb-Exp: 129(59.2) | Self-administered questionnaire | Years of occupational exposure; CA: 11.5[2–28] CO_Asb-Exp: 17.5[5–27.5]Years of environmental exposure;CA: 30[18–42] CO: 35[20–46] | Asbestos; Yes, CA: 104(96.3)CO_Asb-Exp: 199(91.3)No, CA: 4(3.7)CO_Asb-Exp: 19(8.7)Occupational; Yes,CA: 82(75.9) CO_Asb-Exp: 128(58.7)No, CA: 26(24.1) CO_Asb-Exp: 90(41.3)Environmental; Yes, CA: 77(71.3) CO_Asb-Exp: 145(66.5)No, CA: 31(28.7) CO_Asb-Exp: 73(33.5) | Plasma | MPM |
| Faversani 202121 | Italy | Hospital based case-control | N: 51CA_MPM: 23CO: 09CA_Lung-Ad: 14 | CA_MPM; M: 23 F: N/ACO; M: 09, F: N/ACA_Lung-Ad; M: 14, F: N/A | CA_Lung-Ad: 69 (±7)CO: 67 (±7)CA_MPM: NR[55–90] | CA_Lung-Ad;Ex: 10 (71)S: 2 (14.3)NA: 2 (14.3)CO;Ex: 6 (66.7)Yes: 3 (33.3)NA: 0 (0) | NR | NR | NR | EBC and Plasma | MPM, Lung-Ad |
| Weber 201937 | Germany | Hospital based case-control | N: 51CA: 17CO: 34 | CA: 17(100)CO: 34(100) | CA: 73[64 – 83]CO: 74[63 - 84] | CA;S: 1Ex: 13NS: 3CO;S: 4Ex: 21NS: 9 | Self-administered questionnaire | NR | Occupational: NR(100 %) | Plasma | MPM |
| Matboli 201832 | Egypt | Hospital based case-control | N: 100CA: 60CO_Asb-Exp: 20CO_Non_Asb-Exp: 20 | MPM; M: 35(58.3)F: 25(41.7)CO_Asb-Exp; M: 12(60)F: 8(40)CO_Non_Asb-Exp; M: 12(60) F: 8(40) | CA; ≥55 years: 33 (55) <55 years: 27(45)CO_Asb-Exp; ≥55 years: 11(55)<55 years: 9(45)CO_Non_Asb-Exp; ≥55 years: 10(50)<55 years: 10(50) | CA; S: 24 (40)NS: 27 (45) Ex: 8 (13.3) PS: 1 (1.7)CO_Asb-Exp;S: 5 (25)NS: 14 (70) PS: 1 (5)CO_Non_Asb-Exp;S: 6 (30)NS: 13 (65) PS: 1 (5) | NR | NR | CA; +ve exp: 45(76.3)-ve exp: 14(23.7)CO_Asb-Exp;+ve exp: 20 (100)CO_Non_Asb-Exp; -ve exp: 20(100) | Blood serum | MPM |
| Santarelli 201922 | Italy | Hospital based case-control | N: 397CA_NSCLC-Asb: 105CA_NSCLC: 60CO_Asb-Exp: 80CA_MPM: 74CO: 78 | Discovery;CA_NSCLC; M: NR (100)F: 0(0)CA_NSCLC-Asb; M: NR (100)F: 0(0)CA_MPM; M: NR(100)F: 0(0)Serum Training;CO; M: NR(79) F: NR(21)CA_NSCLC; M: NR(60) F: NR(40)CA_NSCLC-Asb; M: NR(80) F: NR(20)CA_MPM; M: NR(83)F: NR(17) | Discovery;CA_NSCLC: 65±7CA_NSCLC-Asb: 69±11CA_MPM: 72±8Serum Training;CO: 56±17CA_NSCLC: 71±10CA_NSCLC-Asb: 74±9CA_MPM: 71±7 | Discovery;CA_NSCLC; NS: NR (25) S: NR (75)CA_NSCLC-Asb; NS: NR (25)S: NR (75)CA; NS: NR (25)Yes: NR (75)Serum Training;CO; NS: NR (42)S: NR (26)Ex: NR (32)CA_NSCLC; NS: NR (29)S: NR (26)Ex: NR (45)CA_NSCLC-Asb; NS: NR (20)S: NR (35), Ex: NR (45)CA_MPM; NS: NR (38)S: NR (12)Ex: NR (50) | Self-administered questionnaire | Discovery set: NRSerum Training;CA_NSCLC-Asb: 6.4(±3.7)CA_MPM: 4.2(±3.3) | Discovery;CA_NSCLC; Not exposed: NR (100)CA_NSCLC-Asb; Occupational: NR (100)CA_MPM; Occupational: NR (100)Serum Training;CO; Not exposed: NR (100)CA_NSCLC; Not exposed: NR (100)CA_NSCLC-Asb; Occupational: NR (45), Environmental: NR (55)CA_MPM; Occupational: NR (59) Environmental: NR (41) | Blood serum | NSCLC, MPM |
| Cavalleri 201723 | Italy | Hospital based case-control | N: 42CA: 23CO_Asb-Exp: 19 | CA; M: 17(73.9)F: 6(26.1)CO_Asb-Exp; M: 15 (78.9)F: 4(21.1) | CA: 70.2(±7.8)CO_Asb-Exp: 66.5(±6.4) | CA; S: 5(21.8)Ex: 12(52.1)NS: 6(26.1)CO_Asb-Exp; S: 3(15.8)Ex: 7(36.8)NS: 9(47.4) | Questionnaire administered via personal interview | NR | CA; Definite Occupational: 12(52.3)Possible Occupational: 2(4.3)Environmental: 2(4.3)NA: 7(30.4)CO_Asb-Exp; Definite Occupational: 19(100) | Plasmatic EV | MPM |
| Mozzoni 201725 | Italy | Hospital based case-control | N: 61CA: 32CO_Asb-Exp: 14CO_Non_Asb-Exp: 15 | CA; M: 24(NR)F: 3(NR)CO_Non_Asb-Exp; M: 12(NR)F: 3(NR)CO_Asb-Exp; M: 8(NR) F: 6(NR) | CA: 72.3(±15.6)CO_Asb-Exp: 75.9(±9.9)CO_Non_Asb-Exp:69.9(±7.0) | CA; NS: 12(NR)S: 20(NR)Ex: 00(NR)CO_Asb-Exp; NS: 8(NR)S: 4(NR)Ex: 2(NR)CO_Non_Asb-Exp; NS: 5(NR)S: 7(NR) Ex: 3(NR) | NR | NR | NR | Plasma and FFPE tissue samples | MPM |
| Weber 201735 | Germany | Hospital based case-control | N: 66CA: 22CO_Asb-Exp: 44 | Discovery;CA; M: 21F: N/ACO_Asb-Exp; M: 21F: N/A | Discovery;CA: 72[35–85]CO_Asb-Exp: 72[43–82] | Discovery;CA; S: 12(NR)NS: 9(NR)CO_Asb-Exp; S: 12(NR)NS: 9(NR) | NR | NR | NR | Plasma | MPM |
| Truini 201724 | Italy | Hospital based case-control | N: 57TS: 27VS: 30 | TS; M: 22(81) F: 5(19)VS; M: 25 (83) F: 5(17) | TS: 67.9(±6.5)VS: 69.5(±8.5) | TS; S: 18(67)NS: 9(33)Unknown: 0(0)VS; S: 19(63)NS: 9(30)NA: 2(7) | NR | NR | TS; Yes: 22(81)NA: 5(19)VS; Yes: 20(67)NA: 10(33) | FFPE biopsy tumourtissue | MPM |
| Bononi 201626 | Italy | Hospital based case-control | N: 30CA: 10CO_Asb-Exp: 10CO_Non_Asb-Exp: 10 | NR | CA: 64[NR]CO_Asb-Exp: 64[NR]CO_Non_Asb-Exp: 64[NR] | NR | NR | NR | NR | Blood serum | MPM |
| Ak 201541 | Turkey | Hospital based case-control | N: 24CA: 18CO_B_Asb-Exp: 06 | MPM; M: 09F: 09CO_B_Asb-Exp; M: 5F: 01 | CA: 68.0(±7.5), NR [48–81]CO_B_Asb-Exp: 65.7(±12.3), NR [49–79] | NR | NR | CA: 33.1(±19.6), NR [0–81]CO_B_Asb-Exp: 28.2(±11.3), NR [20–49] | NR | Fresh frozen tissues | MPM |
| Santarelli 201527 | Italy | Hospital based case-control | N: 188CA: 45CO_Asb-Exp: 99CO_Non_Asb-Exp: 44 | CA; M: 40(89)F: 5(11)CO_Asb-Exp; M: 90(91)F: 9(9)CO_Non_Asb-Exp; M: NR (80)F: NR (20) | CA: 69 (±8)CO_Asb-Exp: 64(±10)CO_Non_Asb-Exp: 68(±6) | CA; S or Ex, No: 14(31)Yes: 31(69)CO_Asb-Exp; S or Ex, No: 39(39) Yes: 61(61)CO_Non_Asb-Exp; S or Ex, No: NR (41) Yes: NR(59) | Self-administered questionnaire | CA: 25(±12)CO_Asb-Exp: 28(±11) | NR | Blood serum | MPM |
| Kirschner 201531 | Australia | Hospital based retrospective cohort study | N: 176EPP: 85P/D: 75LS: 08SS: 08 | EPP; M: 68(80)F: 17(20)P/D; M: 59(79)F: 16 (21)LS; M: 6(NR)F: 2(NR)SS; M: 6(NR)F: 2(NR) | EPP: 58[22–74]P/D: 66[42–83]LS: 51.5[37–64]SS: 62[47 – 70] | NR | NR | NR | NR | FFPE tumor specimens | MPM |
| Lamberti 201528 | Italy | Hospital based case-control | N: 24CA: 14CO_Non_Asb-Exp: 10 | CA; M: 13F: 01CO_Non_Asb-Exp; M: 09 F: 01 | CA: 70.3(±4.6)CO_Non_Asb-Exp: 68.2(±5) | NR | NR | NR | NR | Blood serum | MPM |
| Andersen 201440 | Denmark | Hospital based case-control | N: 71CA: 40CO; CO_DB: 12 CO_NNP: 14CO_PTHX: 5 | CA; M: 32(80)F: 8(20)CO; CO_DB, M: 9(75), F: 3(25)CO_NNP, M: 11(79), F: 3(21)CO_PTHX, M: 5(100), F: 00(00) | CA: 64[40–77]CO; DB: 58.5[43–70]NNP: 65.5[44–72]PTHX: 34[20–38] | CA; S: 26(65)NS: 14(35)CO; DB, S: 8(67), NS: 4(33) NNP, S: 10(71), NS: 4(29)PTHX; S: 1(20), NS: 4(80) | NR | NR | NR | FFPE tumor specimens | MPM |
| Weber 201438 | Germany | Hospital based case-control | N: 95CA: 43CO_Asb-Exp: 52 | CA; M: 43F: N/ACO_Asb-Exp; M: 52F: N/A | CA: 72[35–85]CO_Asb-Exp: 73[43–85] | CA; S: 21(NR)NS: 20(NR)CO_Asb-Exp; S: 34(NR)NS: 18(NR) | NR | NR | NR | Blood - cellular blood fraction | MPM |
| Gayosso-Gómez 201433 | Mexico | Hospital based case-control | N: 92CA_MPM: 11CO_Non_Asb-Exp_1: 23CO_Non_Asb-Exp_2: 22CA _Lung-Ad_1: 15CA _Lung-Ad_2: 21 | CA_MPM; M: 10(NR)F: 1(NR)CA _Lung-Ad_1; M: 04(NR)F: 11(NR) CA _Lung-Ad_2; M: 13(NR)F: 8(NR)CO_Non_Asb-Exp_1; M: 23(NR)F: 00(00)CO_Non_Asb-Exp_2; M: 00(00)F: 22(NR) | CA_MPM: 65.8(±13.4)CA _Lung-Ad_1: 61.7(±11.4) CA _Lung-Ad_2: 60.9(±15.4)CO_Non_Asb-Exp_1: 47.25(±5.8) CO_Non_Asb-Exp_2: 57.0 (±10.0) | CA_MPM; S: 09(NR)NS: 02(NR)CA _Lung-Ad_1; NS: 15(NR) CA _Lung-Ad_2; S: 21(NR)CO_Non_Asb-Exp_1; S: 23(NR)CO_Non_Asb-Exp_2; S: 22(NR) | Questionnaire administered via personal interview | NR | NR | Blood serum | MPM, Lung-Ad |
| Xu 201344 | USA | Hospital based case-control | N: 31CA: 25CO_Non_Asb-Exp: 06 | CA; M: 14(NR)F: 11(NR)CO_Non_Asb-Exp; M: 04(NR)F: 02(NR) | CA: NR [35–70]CO_Non_Asb-Exp: NR [58–71] | NR | NR | NR | NR | Tumor samples specimens and Normal parietal pleura | MPM |
| Muraoka 201334 | Japan | Hospital based case-control | N: 110CA: 48CO_B_Asb-Exp: 21CO_Non_Asb-Exp: 41 | CA; M: 45(94)F: 3(6)CO_B_Asb-Exp; M: 15(71)F: 6(29)CO_Non_Asb-Exp; M: 23(56)F: 18(44) | Overall: 69[38–91]CA; <69 years: 28(58) ≥69 years: 20(42)CO_B_Asb-Exp; <69 years: 4(20)≥69 years: 17 (80)CO_Non_Asb-Exp; <69 years: 23(56) ≥69 years: 18(44) | CA; NS: 14(29)S: 34(71)CO_B_Asb-Exp; NS: 9(43)S: 12(57)CO_Non_Asb-Exp; NS: 23(56)S: 18(44) | NR | NR | NR | Serum | MPM |
| Kirschner 201242 | Australia | Hospital based case-control | N: 94Plasma or serum;CA: 15CO_Non_Asb-Exp: 14Tissue;CA: 18CO_Non_Asb-Exp: 7 | Plasma or SerumCA; M: 13(NR)F: 02(NR)CO_Non_Asb-Exp; M: 09(NR)F: 05(NR)TissueCA; M: 14(NR)F: 04(NR)CO_Non_Asb-Exp; M: 05(NR)F: 02(NR) | Plasma or SerumCA: 68[51 – 83]CO_Non_Asb-Exp: 58.5[21–78]Tissue SamplesCA: 55[37 – 66]CO_Non_Asb-Exp: 68[57 – 76] | NR | NR | NR | NR | Plasma or serum and ling tissue | MPM |
| Weber 201239 | Germany | Hospital based case-control | N: 65CA: 23CO_Asb-Exp: 17CO_Non_Asb-Exp: 25 | CA; M: 18(NR) F: 5(NR)CO_Asb-Exp; M: 16(NR) F: 01(NR)CO_Non_Asb-Exp; M: 18(NR) F: 7(NR) | CA: 66[34–84]CO_Asb-Exp: 68[47–80]CO_Non_Asb-Exp: 70[56–84] | CA; Ex: 10(NR)NS: 12(NR)NA: 01(NR)CO_Asb-Exp; S: 06(NR)Ex: 05(NR)NS: 06(NR)CO_Non_Asb-Exp; S: 05(NR)Ex: 15(NR)NS: 05(NR) | NR | NR | NR | Cellular fraction of human peripheral blood | MPM |
| Tomasetti 201229 | Italy | Hospital based case-control | N: 121CA_MPM: 45CA_NSCLC: 20CO_Non_Asb-Exp: 56 | CA_MPM; M: 31(NR)F: 14(NR)CA_NSCLC; M: 15(NR)F: 5(NR)CO_Non_Asb-Exp; M: 34(NR)F: 22(NR) | CA_MPM: 67.7(± 8.9)CA_NSCLC: 69.6(±8.1)CO_Non_Asb-Exp: 66.0(±6.8) | CA_MPM; S: 21 (47)Ex: 9(20) NS: 15(33)CA_NSCLC; S: 5(25)Ex: 6(30)NS: 9 (45)CO_Non_Asb-Exp; S: 27(48)Ex: 8(14) NS: 21(38) | Self-administered questionnaire | NR | NR | Blood serum | MPM, NSCLC |
| Foss 201130 | Italy | Hospital based case-control | N: 78CA: 03CA_NSCLC: 33CO_Non_Asb-Exp: 42 | Discovery,CO; M: 11F: 00CA_NSCLC; M: 10 F: 01 | Discovery,CO: 64[60–74]CA_NSCLC: 65[50–72] | Discovery CohortCO; NS: 1(NR)S: 5(NR)Ex: 5(NR)CA_NSCLC; S: 6(NR)Ex: 5(NR) | NR | NR | NR | Blood serum | NSCLC, MPM |
| Nymark 201143 | Finland | Hospital based case-control | N: 34CA: 13CO_Asb-Exp: 13CO_Non_Asb-Exp: 8 | CA; M: 13F: 00CO_Asb-Exp; M: 13F: 00CO_Non_Asb-Exp; M: 08F: 00 | CA: 62.5(NR)CO_Asb-Exp: 62.6(NR)CO_Non_Asb-Exp: NR | CA; S: NR(38)Ex: NR(62)CO_Asb-Exp; S: NR(54)Ex: NR(46)CO_Non_Asb-Exp; S: NR(57)Ex: NR(43) | NR | CA: 33.4(NR)CO_Asb-Exp: 0.04(NR)CO_Non_Asb-Exp: 0.7(NR) | NR | FFPE biopsy tumourtissue | MPM |
Abbreviations: CA: cases, CO_Non_Asb-Exp: controls non-exposed to asbestos, MPM: malignant pleural mesothelioma, CO_Asb-Exp: controls exposed to asbestos, exp: exposure, +ve exp: positive exposure, -ve exp: negative exposure, EV: extracellular vesicles, Lung-Ad: lung adenocarcinoma, N: total sample size, SD: Standard deviation, M: male, F: female, S: smoker, NS: non-smoker, Ex: former smoker, NA: data not available, N/A: not applicable, EBC: exhaled breathe condensate, NR: data not reported, NSCLC: non-small cell lung cancer, NSCLC_Asb: Asbestos exposed non-small cell lung cancer, TS: training set, VS: validation set, FFPE: formalin-fixed paraffin embedded, EPP: extra pleural pneumonectomy, P/D: pleurectomy ± decortication, LS: long survivor, SS: short survivor, NNP: patient-matched non-neoplastic pleura, PTHX: non neoplastic reactive mesothelial proliferation due to pneumothorax, BAsb-Exp: Benign pleural asbestosis, Y: years.
MicroRNAs found associated with diagnosis and/or prognosis of asbestos-related LC and/or MPM in the studies included in the systematic review.
| Author, Year | Matrix of miRNA | miRNAs | miRNA Expression change | Statistical model and adjustment for confounders | Effect estimates (95 % CI) of miRNAs expression change | miRNAs expression changes in Fold change (FC) | Adjustment for multiple tests | Cancer type | Cancer Histology | Cancer Stage |
|---|---|---|---|---|---|---|---|---|---|---|
| Casalone 202218 | Serum Extracellular Vesicles | miR-11,400 | ↑ | Multivariable logistic regression model adjusted for age, batch effect, country and asbestos exposure | NR | miR-11,400: 1.4 (0.69–2.0)miR-148a-3p: 0.6 (0.2–0.82)miR-409–3p: 0.7 (0.02–1.3) | miR-11,400: 0.01 (FDR - adjusted p value) | MPM | NR | NR |
| miR-148a-3pmiR-409–3p | ↓ | |||||||||
| Mauro 202319 | Blood serum | miR-197‑3p | ↑ | ANOVA testmatching by age | NR | NR | CA vs. CO_Asb-Exp: 0.0036CO_Asb-Exp vs. CO_Non_Asb-Exp: 0.0001(Tukey test – adjusted p value) | MPM | Epithelioid: 27 (36.0)Sarcomatoid: 20 (26.7)Biphasic: 28 (37.3) | NR |
| Jiménez-Ramírez 202236 | Plasma | miR-103a-3p | ↓ | Mann-Whitney U, Chi-squared or Fisher's exact testMatching by sex and age | NR | NR | NR | MPM | Epithelioid: 102(94.4)Biphasic: 2(1.9)Sarcomatoid: 4 (3.7) | NR |
| Ferrari 202220 | Plasma samples | miR-103a-3p miR-30e-3p | ↓ | Multivariable logistic regression model adjusted for sex, age, BMI, and smoking | OR;miR-103a-3p: 0.99996(0.99970–1.000)miR-30e-3p: 1.00004(0.99950–1.001) | miR-103a-3p: 0.57miR-30e-3p: 0.76 | NR | MPM | Epithelioid: 14(54)Biphasic: 10(38)Sarcomatoid: 2(8) | Stage I: 8(31)Stage II: 6(23)Stage III: 7 (27)Stage IV: 5 (19) |
| Faversani 202121 | Blood plasma | miR-597–5pmiR-1260a | ↑ | Multivariable logistic regression model adjusted for age, BMI and smoking habits | OR;miR-597–5p: 0.852miR-1260a: 1.615miR-130b-3p: 0.410miR-302b-3p: 2.849miR-518f-3p: 1.030let-7f-5p: 0.301miR-345–5p: 0.406miR-362–5p: 0.390miR-1260a: 1.615 | Blood plasma;miR-597–5p: 2.4miR-1260a: 9.9Let-7f-5p: 0.39miR-1260a: 21.5miR-130b-3p: 0.98miR-302b-3p: 0.06miR-345–5p: 2.68miR-362–5p: 2.04miR-518f-3p: 4.0miR-597–5p: 2.57 | let-7f-5p: 0.399miR-1260a: 0.999miR-130b-3p: 0.999miR-302b-3p: NRmiR-345–5p: 0.936miR-362–5p: 0.999miR-518f-3p: 0.200(FDR - adjusted p value) | MPM | Lung_Ad: NR(100) | Stage I - II: NR(100) |
| miR-130b-3p miR-302b-3p miR-518f-3p | ↓ | |||||||||
| let-7f-5p miR-345–5p miR-362–5p | ↓ | Lung-Ad | ||||||||
| miR-1260a | ↑ | MPM | ||||||||
| EBC | miR-1260a | ↑ | ||||||||
| EBC;miR-1260a: 1.26miR-597–5p: 1.42 | ||||||||||
| Weber 201937 | Plasma | miR-132–3pmiR-126–3p miR-103a-3p Combination of three miRNAs | ↓ | Mann–Whitney U tests and Kruskal–Wallis testsMatching by age, sex, smoking status, and date of blood collection | NR | NR | NR | MPM | Epithelioid: 10(58.8)Biphasic: 2(11.8)Sarcomatoid: 3(17.6)Not specified: 2(11.8) | NR |
| Matboli 201832 | Blood serum | miR-548a-3p miR-20a | ↑ | Kruskal–Wallis tests and one way analysisMatching by sex, age, history of smoking and asbestos exposure | Standardized coefficients (β) Linear regression analysismiRNA‐2053 after cutoff: 0.303(0.072–0.522) | CA;miR-250,053: 19.3729CO_Asb-Exp;miR-250,053: 0.8450CO_Non_Asb-Exp;miR-250,053: 0.7492 | NR | MPM | NR | Stage I: 46(76.7)Stage II: 13 (21.7)Stage III: 1 (1.7) |
| Santarelli 201922 | Blood serum | miR-126miR-222 | ↑ | Multivariable logistic regression model adjusted for age, sex, and smoking | OR;miR-222: 1.501 (1.148–1.958)miR-222/miR-126: 0.138(0.019–0.076) | CA_NSCLC;miR-126: 0.90miR-205: 2.93miR-222: 1.84miR-520 g: 1.09CA_NSCLC-Asb;miR-126: 1.18miR-205: 2.91miR-222: 4.14miR-520 g: 0.63CA;miR-126: 0.45miR-205: 0.61miR-222: 0.85miR-520 g: 0.65 | miR-222: 0.003miR-222/miR-126: 0.047(two-tailed Student t-test - adjusted p value) | MPM, NSCLC | Discovery,CA_NSCLC; Adenocarcinoma: NR(100)CA_NSCLC_Asb; Squamous: NR(25) Adenocarcinoma: NR(75)CA; Epithelioid: NR(90) Biphasic: NR(10)Serum_TrainingCA_NSCLC; Squamous: NR(35) Large cell: NR(10) Adenocarcinoma: NR(55)CA_NSCLC_Asb; Squamous: NR(36) Large cell: NR(18) Adenocarcinoma: NR(46)CA; Epithelioid: NR(75) Sarcomatoid: NR(25) | NR |
| Cavalleri, 201723 | Plasmatic extracellular vesicles (Evs) | miR-103amiR-98miR-148bmiR-744miR-30e-3p | ↓ | Cox multivariableregression model adjusted for age, sex, BMI and smoking. | HR;miR-103a: 0.37(0.13–1.13)miR-30e-3p: 0.51(0.17 - 1.52) | miR-103: 0.25miR-98: 0.23miR-148b: 0.29miR-744: 0.31miR-30e-3p: 0.37 | miR-103: 0.056miR-98: 0.056miR-148b: 0.056miR-744: 0.056miR-30e-3p: 0.056(FDR - adjusted p value) | MPM | Epithelioid: 10(NR)Biphasic: 11(NR)Sarcomatoid: 01(NR)Not specified: 01(NR) | NR |
| Mozzoni 201725 | Blood plasma and FFPE tissue | miR-17miR-126 miR-16 miR-486 | ↓ | Two-sided, two-sample t-testNR | NR | NR | NR | MPM | Epithelioid: 26(NR)Biphasic: 6(NR) | Stage I: 2(NR)Stage II: 9(NR)Stage III: 15(NR)Stage IV: 6(NR) |
| Weber 201735 | Plasma | miR-132–3p | ↓ | Wilcoxon rank-sum testsMatching by age and smoking | NR | NR | NR | MPM | Discovery;Epithelioid: 14(NR)Biphasic: 4(NR)Sarcomatoid: 3(NR)Not specified: 0(NR) | NR |
| Truini 201724 | FFPE biopsy tumor | miR-99alet-7cmiR-125b | ↓ | Cox multivariable regression model adjusted for age and histological subtype | HR;Training setmiR-99a: 0.42(NR)let-7c: 0.32 (NR)miR-125b: 0.41 (NR)TCGA MPM datasetmiR-99a-5p: 0.75(NR)let-7c: 0.79(NR)miR-125b-5p: 0.63(NR) | miR-99a-5p: 0.14let-7c: 0.18miR-125b-5p: 0.31 | Training set;miR-99a: 0.0014 let-7c: 0.0014miR-125b: 0.0010TCGA MPM datasetmiR-99a-5p: 0.0040let-7c: 0.0240miR-125b-5p: 0.0010(Cox proportional hazard – adjusted p value) | MPM | Epithelioid: 20(74) Sarcomatoid: 3(11) Biphasic: 3 (11) Not typified: 1(4) | NR |
| Bononi 201626 | Blood serum | miR-197–3pmiR-1281miR 32–3pmiR-197–3pmiR-32–3pmiR-1281 | ↑ | NR | NR | miR-1281: 3.7miR-32–3p: 18miR-197–3p; CA vs. CO_Non_Asb-Exp: 4.4CA vs. CO_Asb-Exp: 5.5miR-1281; CA vs. CO_Non_Asb-Exp: 3.7CO_Asb-Exp vs. CO_Non_Asb-Exp: 3.3miR-32–3p; CA vs CO_Non_Asb-Exp: 17.6CO_Asb-Exp vs. CO_Non_Asb-Exp: 61.5miR-197–3p; CA vs. CO_Non_Asb-Exp: 1.8CA vs. CO_Asb-Exp: 2.5miR-1281; CA vs. CO_Non_Asb-Exp: 2.5CA vs. CO_Asb-Exp: 3.5 | NR | MPM | NR | NR |
| Ak 201541 | Fresh frozen tissues | miR-484miR-320let-7amiR-744miR-20amiR-193blet-7dmiR-125a-5pmiR-92amiR-155miR-152 | ↑ | NR | NR | miR-484: 5.58miR-320: 2.87let-7a: 13.93miR-744: 4.26miR-20a: 5.7miR-193b: 3.03let-7d: 5.82miR-125a-5p: 8.17miR-92a: 2.39miR-155: 3.16miR-152: 2.93 | miR-484: 0.010miR-320: 0.017let-7a: 0.019miR-744: 0.019miR-20a: 0.019miR-193b: 0.019let-7d: 0.045miR-125a-5p: 0.045miR-92a: 0.045miR-155: 0.045miR-152: 0.047(Multiple hypothesis testing, q value function – adjusted p value) | MPM | Epithelial:10(55.6)Mixed: 4(22.2)Sarcomatoid: 4(22.2) | Stage I - II: 4 (22.2)Stage III - IV: 14 (77.8) |
| Santarelli 201527 | Blood serum | miR-126 | ↓ | Multivariable regression modeladjusted for age, sex, smoking and asbestos exposure | CO_Non_Asb-Exp vs CO_Asb-Exp;miR-126: 1.23(1.0–1.6) (p = 0.056)CO_Asb-Exp vs. CA;MiR-126: 1.13(0.9–1.4) (p = 0.259)CA vs. CO_Non_Asb-Exp;MiR-126: 1.34(1.0–1.8) (p = 0.05) | NR | NR | MPM | Epithelioid: 33 (73 %)Biphasic: 9 (20 %)Sarcomatoid: 3 (7 %) | NR |
| Kirschner 201531 | Formalin-fixed paraffin embedded (FFPE) tumor specimens | miR-21–5pmiR-23a-3pmiR-30e-5pmiR-221–3pmiR-222–3p miR-31–5p | ↓ | Multivariable logistic regression model adjusted for histological subtype, age, and sex | OR;miR-21–5p: 0.87miR-23a-3p: 1.20miR-30e-5p: 0.79miR-221–3p: 0.79miR-222–3p: 0.90miR-31–5p: 1.06HR from Cox regression model:Univariable model:miR-21–5p: 2.84(1.48–5.45)Multivariable model:hsa-miR-21–5p: 3.35(1.66–6.75) | miR-222–3p: −4.43miR-221–3p: −3.51miR-210–3p: −3.46miR-21–5p: −2.76miR-93–5p: −2.67miR-106b-5p: −2.51miR-17–5p: −2.50miR-27a-3p: −2.16miR-20a-5p: −2.12miR-19b-3p: −2.02miR-30e-5p: −2.02miR-23a-3p: −1.81miR-662: 2.88Microarray only;miR-1469: 2.02miR-1826: 2.29miR-298: 15.39RT-qPCR only;miR-29c-5p: NAmiR-31–5p: NAmiR-106a-5p: NAmiR-126–3p: NAmiR-625–3p: NAmiR-92a-3p: NAmiR-24–3p: NART-qPCR ResultsMicroarray and RT-qPCR;miR-222–3p: −2.7miR-221–3p: −2.49miR-210–3p: −2.68miR-21–5p: −1.76miR-93–5p: −2.29miR-106b-5p: −2.21miR-17–5p: −2.23miR-27a-3p: −1.79miR-20a-5p: −1.82miR-19b-3p: −1.49miR-30e-5p: −2.34miR-23a-3p: −1.73miR-662: 1.14RT-qPCR;miR-29c-5p: 1.03miR-31–5p: 1.51miR-106a-5p: −2.00miR-126–3p: −1.75miR-625–3p: −1.11miR-92a-3p: −1.39miR-24–3p: 1.51 | miR-221–3p: 0.0147miR-210–3p: 0.0465miR-21–5p: 0.0465miR-93–5p: 0.0495miR-27a-3p: 0.0465(Benjamini-Hochberg Correction for FDR – adjusted p value) | MPM | EPP - Complete cohort; Epithelioid: 65(76) Biphasic: 20(24) Sarcomatoid: 0(0)P/D - Complete cohort; Epithelioid: 37(49) Biphasic: 26(35) Sarcomatoid: 12(16) | NR |
| Lamberti 201528 | Blood serum | miR-101miR-25miR26bmiR335miR433 | ↑ | NR | NR | NR | NR | MPM | Epithelial: 07(NR)Sarcomatoid: 03(NR)Mixed: 04(NR) | Stage I: 05(NR)Stage II: 03(NR)Stage III: 06(NR) |
| miR191miR-223 | ↓ | |||||||||
| Andersen 201440 | Formalin-fixed, paraffin-embedded tissue specimens | miR-378miR-365amiR-193a-3pmiR-193bmiR-210 | ↑ | Multivariable logistic regression model used to estimate the performance of each miRNA (i.e., miR-143, miR-145, and miR-652) adjusted for the effect of the other 2 miRNAs | Estimate (where p< 0.001)Intercept: 4.38(4.22–4.54)miR-126: 0.53(0.49–0.57)miR-143: 0.98(0.94–1.02)miR-145: −2.34[(−2.27) – (−2.39)]miR-652: −1.45 [(−1.40) – (−1.50)] | CO_DB vs CAmiR-126: 1.95miR-143: 2.44miR-145: 1.54miR-193a-3p: 2.49miR-193b: 1.31miR-652: 1.20CA vs CO_NNPmiR-126: −2.91miR-143: −2.62miR-145: −6.95miR-193a-3p: 2.38miR-193b: −1.90miR-652: −3.06 | CO_DB;miR-126: −1.91 (1.53)miR-143: −1.32 (1.11)miR-145: −1.91 (1.33)miR-193a-3p: −2.43 (1.20)miR-193b: −0.37 (0.66)miR-652: 1.60 (1.12)CO_NNP;miR-126: −4.42 (1.53)miR-143: −4.00 (1.64)miR-145: −5.33 (1.30)miR-193a-3p: −2.36 (1.32)miR-193b: 0.95 (1.12)miR-652: −0.27 (1.11)CA;miR-126: −2.87 (1.28)miR-143: −2.61 (1.27)miR-145: −2.53 (1.42)miR-193a-3p: −1.11 (1.13)miR-193b: 0.02 (1.24)miR-652: 1.34 (0.87)CO_PTHX;miR-126: −5.50 (1.40)miR-143: −4.13 (1.49)miR-145: −4.79 (1.27)miR-193a-3p: −2.38 (0.76)miR-193b: −1.14 (0.74)miR-652: 0.30 (1.17)turky-kramer post hoc test – adjusted p value | MPM | MPM; Epithelioid: 18(45), Biphasic: 22(55)CO_DB; Epithelioid: 9(75), Biphasic: 3(25) | CA,Stage I: 1(3)Stage II: 6(15)Stage III: 23(58)Stage IV: 10(25)CO_DB,Stage I: 1(8)Stage II: 8(67)Stage III: 3(25) |
| let-7cmiR-99a miR-126miR143miR-145miR-144–5pmiR-451amiR-486–5pmiR-652 | ↓ | |||||||||
| Weber 201438 | Blood - cellular blood fraction | miR-103a-3p | NR | NR | NR | NR | NR | MPM | Epithelioid: 28(NR), Biphasic: 6(NR), Sarcomatoid: 5(NR), Not specified: 4(NR) | NR |
| Gayosso-Gómez 201433 | Blood serum | miR-1292–5pmiR-409–5pmiR-92b-5pmiR-4791miR-185–5pmiR-96–5pmiR-1271–5p | ↑ | NR | NR | CA vs. CO_Non_Asb-ExpmiR-4791: 8.93miR-185–5p: 3.995miR-96–5p: 4.453miR-1271–5p: InfmiR-1292–5p: InfmiR-409–5p: InfmiR-92b-5p: 6.33CA _Lung-Ad vs CO_Non_Asb-ExpmiR-4791: 10.1449miR-185–5p: 5.1217miR-1271–5p: InfCA vs. CA _Lung-AdmiR-1292–5p: 2.345miR-409–5p: 2.603 | NR | MPM | CA;Epithelioid: 11(NR) | CA, IIIA: 01(NR), IIIB: 03(NR), IV: 04(NR), IIA: 04(NR), IIA: 01(NR), NR: 03(NR)CA _Lung-Ad; IIIB: 05, IV: 31 |
| Xu 201344 | Tumor | miR-551bmiR-483–5pmiR-206miR-363 | ↑ | NR | NR | miR-363: 23.8miR-130b: 3.5miR-221: 3.7miR-155: 3.8miR-21: 3.9miR-379: 4.1miR-629: 7.6 | miR-363: 9.3E-06miR-130b: 1.9E-04miR-221: 4.1E-03miR-155: 1.0E-03miR-21: 1.7E-03miR-379: 1.8E-02miR-629: 9.7E-05(Benjamini-Hochberg Correction for FDR – adjusted p value) | MPM | Epithelial: 18(NR)Biphasic: 4(NR)Sarcomatoid: 3(NR) | NR |
| miR-323–3pmiR-34bmiR-514miR-130bmiR-221miR-155miR-21 | ↓ | |||||||||
| Muraoka 201334 | Serum | miR-34b/c | ↑ | NR | NR | NR | NR | Advanced MPM | Epithelioid: 36(75)Biphasic: 8(17)Sarcomatoid: 4(8) | Stage I: 12(25)Stage II: 5(10)Stage III: 16(33)Stage IV: 12(25)Unknown: 3(7) |
| Kirschner 201242 | Blood plasma | miR-29c*miR-92amiR-625–3p | ↑ | NR | NR | Plasma;miR-29c: 1.64In tumors;miR-625–3p: 4.35miR-29c: −2.65miR-92a: −1.8 | NR | MPM | Test Cohort – CA;Epithelioid: 9(60)Biphasic: 3(20)Sarcomatoid: 2(13.33)Unspecified: 1(0.07)Tissue samples;Epithelioid: 15(83.33)Biphasic: 3(16.66)Sarcomatoid: 0 | NR |
| Weber 201239 | Cellular fraction of human peripheral blood | miR-103miR-20a | ↓ | Mann-Whitney unpairedtestAge, sex, and smoking status were matched | NR | NR | NR | MPM | NR | NR |
| Tomasetti 201229 | Blood serum | miR-126 | ↓ | One-way ANOVA to evaluate differences among MM and NSCLC patients and healthy controls. | NR | NR | Post-hoc Bonferroni test | MPMNSCLC | NR | NR |
| Foss 201130 | Blood serum | miR-1254miR-574–5p | ↑ | NR | NR | NR | miR-574–5p: 0.22miR-1254: 0.42(FDR – adjusted p value) | Early-stage NSCLC | CA_NSCLC, Adenocarcinoma: 6(NR), Bronchioloalveolar carcinoma: 1(NR), Squamous cell carcinoma: 1(NR), Large cell carcinoma: 1(NR), Other: 1(NR), Unavailable: 1(NR) | Discovery,NSCLC,Stage I: 4Stage I/II: 0Stage II: 6Unavailable: 1MPMStage I: 1Stage I/II: 0Stage II: 2Unavailable: 0 |
| Nymark 201143 | Tissues | miR-148b miR-374a miR-24–1* let-7dlet-7emiR-199b-5pmiR-331–3p miR-96 | ↑ | NRAge, sex, nationality, smoking history and distribution of histological types were matched | NR | miR-148b: 0.325miR-374a: 0.5335714miR-24–1*: 0.19904763 | miR-148b: 0.028543miR-374a: 0.028543miR-24–1*: 0.047273 | MPM | Exposed PatientsLCLC: 03AC: 06SCC: 03SCLC: 02Non-exposed patientsLCLC: 01AC: 05SCC: 06SCLC: 02 | NR |
| miR-939miR-671–5pmiR-605miR-1224–5pmiR-202 | ↓ | CO_Asb-Exp;miR-939: −1.2571429miR-671–5p: −0.42619047miR-605: −0.21642858miR-1224–5p: −0.52761906miR-202: −0.632619Lung-AdenocarcinomaCO_Asb-Exp;miR-202: −1.142 | miR-939: 0.018745miR-671–5p: 0.018745miR-605: 0.018745miR-1224–5p: 0.018745miR-202: 0.047273Lung-AdCO_Asb-Exp;miR-199b-5p: 0.049309miR-374a: 0.057633let-7d: 0.067007let-7e: 0.067007miR-331–3p: 0.067007miR-96: 0.067007miR-24–1: 0.067007Lung-AdCO_Asb-Exp;miR-202: 0.049309(Benjamini-Hochberg test, FDR – adjusted p value) |
Abbreviations: CA: cases, CO_Non_Asb-Exp: controls non-exposed to asbestos, MPM: malignant pleural mesothelioma, CO_Asb-Exp: controls exposed to asbestos, exp: exposure, +ve exp: positive exposure, -ve exp: negative exposure, EV: extracellular vesicles, Lung-Ad: lung adenocarcinoma, N: total sample size, M: male, F: female, S: smoker, NS: non-smoker, Ex: former smoker, NA: data not available, N/A: not applicable, EBC: exhaled breathe condensate, NR: data not reported, NSCLC: non-small cell lung cancer, NSCLC_Asb: Asbestos exposed non-small cell lung cancer, TS: training set, VS: validation set, FFPE: formalin-fixed paraffin embedded, EPP: extra pleural pneumonectomy, P/D: pleurectomy ± decortication, LS: long survivor, SS: short survivor, NNP: patient-matched non-neoplastic pleura, PTHX: non neoplastic reactive mesothelial proliferation due to pneumothorax, BAsb-Exp: Benign pleural asbestosis, Y: years, OR: Odds ratio, HR: Hazard ratio, (β): β, standard co-efficient.
Diagnostic and prognostic accuracy of the miRNAs significantly associated with increased lung cancer risk in the included studies.
| Author, Year | miRNA(s) | Sensitivity (%) | Specificity (%) | Sensibility specificity at cut-off | Cut off Value | Positive predictive value (PPV) | Negative predictive value (NPV) | Areas under the curve (AUC) and (95 % CI) for diagnosis | Areas under the curve (AUC) and (95 % CI) for survival (months) | Cancer Histology | Cancer Stage |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Casalone 202218 | miR-11,400miR-148a-3pmiR-409–3p | miR-11,400miR-148–3p and miR-409Discovery set (Prospective): 75 %Validation (Retrospective): 53 % | miR-11,400 miR-148–3pand miR-409Discovery set (Prospective): 70 %Validation (Retrospective): 95 % | NR | NR | miR-11,400miR-148–3p and miR-409Discovery set (Prospective): 71 %Validation (Retrospective): 94 % | miR-11,400 miR-148–3p and miR-409Discovery set (Prospective): 74 %Validation (Retrospective): 57 % | miR-11,400miR-148–3p and miR-409Discovery set (Prospective): 81 %Validation (Retrospective): 86 % | NR | NR | NR |
| Mauro 202319 | miR-197‑3p | NR | NR | NR | NR | NR | NR | ddPCR analyses; miR-197–3p,CA vs. CO_Asb-Exp: 0.65(0.563–0.739)CA vs CO_Non_Asb-Exp: 0.55(0.454–0.640)RT-qPCR analyses; miR-197–3p,CA vs. CO_Asb-Exp: 0.62(0.525–0.708)CA vs CO_Non_Asb-Exp: 0.56(0.472–0.657) | Epithelioid: 13.5(±0.6)Sarcomatoid: 7.9(±0.7)Biphasic: 12.4(±0.6)TOT: 11.5(±0.6) | Epithelioid: 27 (36.0)Sarcomatoid: 20 (26.7)Biphasic: 28 (37.3) | NR |
| Jiménez-Ramírez 202236 | miR-103a-3p | miR-103a-3p;M: 4.4 %F: 0 % | miR-103a-3p;M: 95.5 %F: 97.4 % | NR | miR-103a-3p;M: 1782F: 3082 | M;TP: 4TN: 171F;TP: 00TN: 39 | M;FP: 8FN: 86F;FP: 0FN: 18 | miR-103a-3p;M: 0.426(0.355–0.497)F: 0.437(0.284–0.589) | NR | Epithelioid: 102(94.4)Biphasic: 2(1.9)Sarcomatoid: 4 (3.7) | NR |
| Weber 201937 | miR-132–3pmiR-126–3p miR-103a-3p Combination of three miRNAs | miR-132–3p: 71 %miR-126–3p: 59 %miR-103a-3p: 82 %Combination of three miRNAs: 82 % | miR-132–3p: 47 %miR-126–3p: 72 %miR-103a-3p: 42 %Combination of three miRNAs: 47 % | NR | NR | NR | NR | miR-132–3p: 0.542(0.370–0.713)miR-126–3p: 0.614(0.439–0.789)miR-103a-3p: 0.603(0.440–0.765)Combination of three miRNA (s): 0.605(0.445–0.765) | NR | Epithelioid: 10(58.8)Biphasic: 2(11.8)Sarcomatoid: 3(17.6)Not specified: 2(11.8) | NR |
| Matboli 201832 | miR-548a-3pmiR-20aCombination miRNAs | miR 548a-3p: 91.7 %mir-20a: 96.7 %Combined (mir20a + miR-548a-3p): 100 % | miR 548a-3p: 97.5 %mir-20a: 95.0 %Combined (mir20a + miR-548a-3p): 87.5 % | NR | miRNA-548a-3p: 1.69miRNA-20a: 1.2Combind miRNAs: 1.7 | miR 548a-3p: 98.2 %mir20a: 96.7 %Combined (mir20a + miR-548a-3p): 100 % | miR 548a-3p: 88.6 %mir20a: 95.0 %Combined (mir20a + miR-548a-3p): 92.3 % | miR-548a-3p: 0.922 (0.855–0.982)miR-20a: 0.98 (0.927–0.992)Combind miRNAs: 0.96 (0.917–0.996) | NR | NR | Stage I: 46(76.7)Stage II: 13 (21.7)Stage III: 1 (1.7) |
| Santarelli 201922 | miR-126miR-205 miR-222miR-520 g | miR-222: 80 % | miR-222: 70 % | NR | miR-222: 0.466 | NR | NR | miR-222: 0.767(0.675–0.858) | NR | Discovery,CA_NSCLC; Adenocarcinoma: NR (100)CA_NSCLC_Asb; Squamous: NR(25) Adenocarcinoma: NR(75)CA; Epithelioid: NR(90) Biphasic: NR(10)Serum_TrainingCA_NSCLC; Squamous: NR(35) Large cell: NR(10) Adenocarcinoma: NR(55)CA_NSCLC_Asb; Squamous: NR(36) Large cell: NR(18) Adenocarcinoma: NR(46)CA; Epithelioid: NR(75) Sarcomatoid: NR(25) | NR |
| Cavalleri 201723 | miR-103amiR-98miR-148b miR-744miR-30e-3p | miR-103: 1.000 miR-98: 1.000miR-148b: 1.000miR-744: 0.727miR-30e-3p: 0.636 | miR-103: 0.667miR-98: 0.667miR-148b: 0.733miR-744: 0.867miR-30e-3p: 0.933 | NR | NR | NR | NR | miR-103: 0.864(0.724–1.000)miR-98: 0.864(0.727–1.000)miR-148b: 0.852(0.699–1.000)miR-744: 0.845(0.705–0.986)miR-30e-3p: 0.827(0.679–0.976) | NR | Epithelioid: 10(NR)Biphasic: 11(NR)Sarcomatoid: 01(NR)Not specified: 01(NR) | NR |
| Mozzoni 201725 | miR-17miR-126 miR-16miR-486 | NR | NR | miR-17: 80.0–84.4miR-126: 80.0–97.8miR-486: 80.0–89.1miR-16: 86.7–82.2 | miR-17: 5.9miR-126: 5.4miR-486: 9.2miR-16: 77.5 | NR | NR | miR-17: 0.88(0.78–0.98)miR-126: 0.95(0.89–1.00)miR-486: 0.88(0.79–0.96)miR-16: 0.89(0.81–0.97) | NR | Epithelioid: 26(NR)Biphasic: 6(NR) | Stage I: 2(NR)Stage II: 9(NR)Stage III: 15(NR)Stage IV: 6(NR) |
| Weber 201735 | miR-132–3p | miR-132–3p: 86 %Combination of miR-132–3p with the previously described miR-126; sensitivity: 77 % | miR-132–3p: 61 %Combination of miR-132–3p with the previously described miR-126; specificity: 77 % | NR | NR | NR | NR | Discovery group;miR-132–3p: 0.91(0.80–1.00)Verification group;miR-132–3p: 0.75(0.63–0.88) | NR | Discovery;Epithelioid: 14(NR)Biphasic: 4(NR)Sarcomatoid: 3(NR)Not specified: 0(NR) | NR |
| Ak 201541 | miR-484miR-320 let-7amiR-744 miR-20a miR-193b let-7dmiR-125a-5p miR-92amiR-155 miR-152 | miR-320: ≤7.27let-7a: ≤11miR-125a-5p: ≤9.36 | miR-320: 78 % - 100 %let-7a: 94 % - 83 %miR-125a-5p: 89 % - 100 % | NR | NR | NR | NR | miR-484: ≥0.90 (NR)miR-320: ≥0.90 (NR)let-7a: ≥0.90 (NR)miR-125a-5p: ≥0.90 (NR)miR-484: ≤8.15 (NR) | NR | Epithelial:10(55.6)Mixed: 4(22.2)Sarcomatoid: 4(22.2) | Stage I - II: 4 (22.2)Stage III - IV: 14 (77.8) |
| Santarelli 201527 | miR-126 | miR-126: 75 %Met-TM: 60 %SMRPs: 60 % | miR-126: 54 %Met-TM: 82 %SMRPs: 89 % | NR | SMRPs: 1 (nmol/L)miR-126: 10×10–3 (relative exp)Met-TM: 1 (relative exp) | NR | NR | SMRPs: 0·818(0·723–0·914)miR-126: 0·710(0·568–0·822)Met-TM: 0·750 (0·641–0·858) | NR | Epithelioid: 33 (73 %)Biphasic: 9 (20 %)Sarcomatoid: 3 (7 %) | NR |
| Kirschner 201531 | miR-21–5p miR-23a-3p miR-30e-5p miR-221–3p miR-222–3p miR-31–5 | Combined 6 miR-Score: 82.4 % | Combined 6 miR-Score: 80.6 % | NR | NR | NR | NR | Combined 6 miR-Score: 0.867(0.76–0.96) | EPP (median): 18.86 [0.07–122.41]P/D(median): 7.62 [0.33–224.82]LS (median): 57.2[45.83–90.48]SS (median): 6.4[1.94–8.28] | EPP - Complete cohort; Epithelioid: 65(76) Biphasic: 20(24) Sarcomatoid: 0(0)P/D - Complete cohort; Epithelioid: 37(49) Biphasic: 26(35) Sarcomatoid: 12(16) | NR |
| Andersen 201440 | miR-126miR-143 miR-145 miR-193a-3p miR-193bmiR-652 | All miRNAs (not individual): 0.95 (95 % CI, 0.89 - 1.00) | All miRNAs (not individual): 0.93 (95 % CI, 0.87 - 1.00) | NR | NR | NR | NR | miR-126: 0.78 (0.64 - 0.92)miR-143: 0.76 (0.61 - 0.90)miR-145: 0.93 (0.85 - 1.00)miR-193a-3p: NAmiR-193b: NAmiR-652: 0.89 (0.80 - 0.90) | NR | MPM; Epithelioid: 18(45), Biphasic: 22(55)CO_DB; Epithelioid: 9(75), Biphasic: 3(25) | CA,Stage I: 1(3)Stage II: 6(15)Stage III: 23(58)Stage IV: 10(25)CO_DB,Stage I: 1(8)Stage II: 8(67)Stage III: 3(25) |
| Weber 2014 38 | miR-103a-3p | All subjects;miR-103a-3p: 86 %Epithelioid mesothelioma; miR-103a-3p: 74 %Biphasic mesothelioma; miR-103a-3p: 89 % | All subjectsmiR-103a-3p: 63 %Epithelioid mesothelioma; miR-103a-3p: 85 %Biphasic mesothelioma; miR-103a-3p: 63 % | miR-103a-3pFPR =4 %, Cut-off = 2.01 nmol/lMaximum YI: 749.61FPR = 4 %, Cut off = 99.73Without sarcomatoid mesothelioma, Maximum YI, Cut off = 749.61 | All subjectsmiR-103a-3p: 749.61 | All subjects, miR-103a-3pTP: 37TN: 33 | All subjects, miR-103a-3pFP: 19FN: 6 | miR-103a-3p: 0.76 (NR) | NR | Epithelioid: 28(NR), Biphasic: 6(NR), Sarcomatoid: 5(NR), Not specified: 4(NR) | NR |
| Muraoka 201334 | miR-34b/c | miR-34b/c: 67 % | miR-34b/c: 77 % | NR | NR | NR | NR | miR-34b/c: 0.77 | NR | Epithelioid: 36(75)Biphasic: 8(17)Sarcomatoid: 4(8) | Stage I: 12(25)Stage II: 5(10)Stage III: 16(33)Stage IV: 12(25)Unknown: 3(7) |
| Kirschner 201242 | miR-29c*miR-92amiR-625–3p | CA;miR-625–3p: 73.33 %CO;miR-625–3p: 70 % | CA;miR-625–3p: 78.57 %CO;miR-625–3p: 90 % | NR | NR | NR | NR | CA;miR-625–3p: 0.824 (0.669 - 0.979)CO;miR-625–3p: 0.793 (0.657 - 0.930) | NR | Test Cohort – CA;Epithelioid: 9(60)Biphasic: 3(20)Sarcomatoid: 2(13.33)Unspecified: 1(0.07)Tissue samples;Epithelioid: 15(83.33)Biphasic: 3(16.66)Sarcomatoid: 0 | NR |
| Weber 201239 | miR-103, miR-20a | CO_Asb-Exp: 83 %CO_Non_Asb-Exp: 78 % | CO_Asb-Exp: 71 %CO_Non_Asb-Exp: 76 % | NR | miR-103: 0.621 | NR | NR | miR-103, CA vs. CO_Asb-Exp: 0.757 (95 % CI: 0.586–0.929)miR-103, CA vs. CO_Non_Asb-Exp: 0.871 (95 %CI 0.766–0.977) | NR | NR | NR |
| Tomasetti 201229 | miR-126 | miR-126: 80 % | miR-126: 60 % | NR | NR | NR | NR | CO_Non_Asb-Exp Vs. CA:0.894(0.821–0.968)CO_Non_Asb-Exp Vs. CA_NSCLC: 0.675 (0.503–0.847)CA Vs. CA_NSCLC: 0.751(0.616–0.886) | NR | NR | NR |
| Foss 201130 | miR-1268, miR-574–5p, miR-1254, miR-1258 | NSCLC,miR-1254: 82 %miR-574–5p: 82 %Discovery cohort,miR-1254: 73 %miR-574–5p: 73 % | NSCLC,miR-1254: 77 %miR-574–5p: 77 %Discovery cohort,miR-1254: 71 %miR-574–5p: 71 % | NR | NR | NR | NR | Discovery cohort, miR-1254 and miR-574–5p [miR-1254 + miR-574–5p] = 0.77Validation cohort, miR-1254 and miR-574–5p [miR-1254 + miR-574–5p] = 0.75 | NR | CA_NSCLC, Adenocarcinoma: 6(NR), Bronchioloalveolar carcinoma: 1(NR), Squamous cell carcinoma: 1(NR), Large cell carcinoma: 1(NR), Other: 1(NR), Unavailable: 1(NR) | Discovery,NSCLC,Stage I: 4Stage I/II: 0Stage II: 6Unavailable: 1MPMStage I: 1Stage I/II: 0Stage II: 2Unavailable: 0 |
Abbreviations: CA: cases, CO_Non_Asb-Exp: controls non-exposed to asbestos, MPM: malignant pleural mesothelioma, CO_Asb-Exp: controls exposed to asbestos, exp: exposure, +ve exp: positive exposure, -ve exp: negative exposure, EV: extracellular vesicles, Lung-Ad: lung adenocarcinoma, N: total sample size, M: male, F: female, S: smoker, NS: non-smoker, Ex: former smoker, NA: data not available, N/A: not applicable, EBC: exhaled breathe condensate, NR: data not reported, NSCLC: non-small cell lung cancer, NSCLC_Asb: Asbestos exposed non-small cell lung cancer, TS: training set, VS: validation set, FFPE: formalin-fixed paraffin embedded, EPP: extra pleural pneumonectomy, P/D: pleurectomy ± decortication, LS: long survivor, SS: short survivor, NNP: patient-matched non-neoplastic pleura, PTHX: non neoplastic reactive mesothelial proliferation due to pneumothorax, BAsb-Exp: Benign pleural asbestosis, Y: years.
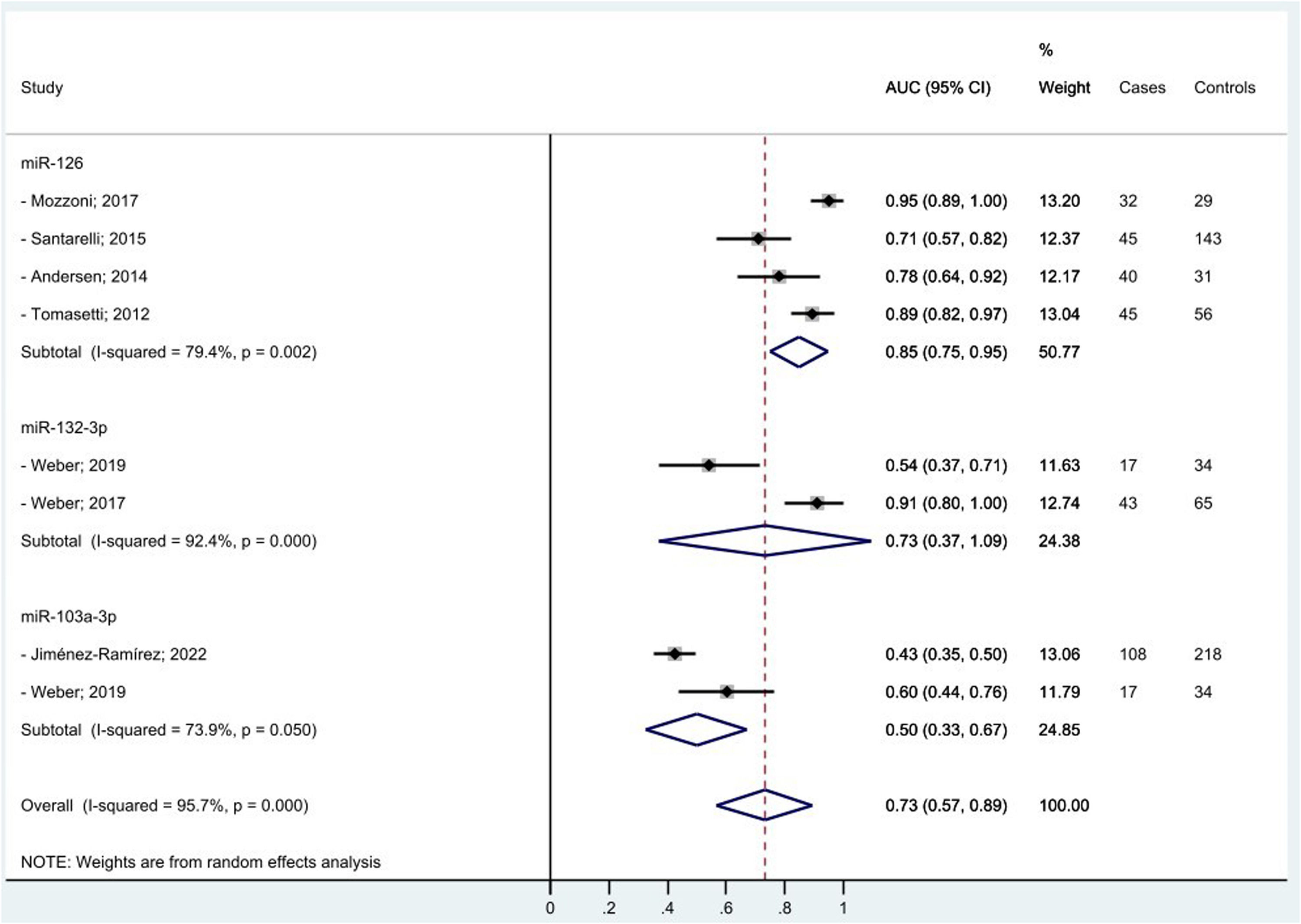
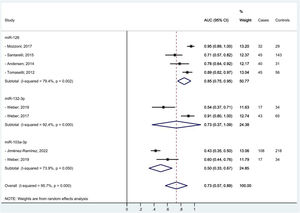
We managed to perform a meta-analysis for miRNAs diagnostic accuracy by pooling the AUC reported by seven similar studies that found the same miRNAs associated to MPM diagnosis among men only.25,27,29,35-37,40 One study,37 contributed twice to the meta-analysis for two different mi-RNAs. Random effect methods were used given the high heterogeneity detected (>50 %). We estimated for miR-126, miR-132–3p, and miR-103a-3p pooled diagnostic AUCs of 85 %, 73 %, and 50 %, respectively. The overall pooled accuracy resulted 73 % (Fig. 2). No small study effect bias was shown in the funnel plot (see supplementary file Fig. 1s) as confirmed by the Egger test (p-value= 0.450).
DiscussionIn our systematic review and meta-analysis to evaluate the role of miRNAs as potential diagnostic and/or prognostic biomarkers of asbestos-related LC and MPM, we found that several miRNAs are promising candidates especially for MPM diagnosis. In particular, we managed to estimate for the three top miRNAs (miR-126, miR-132–3p, and miR-103a-3p) associated to MPM diagnosis a pooled accuracy of 73 % with the highest performance of 85 % for miR-126. In relation to MPM survival, miR-197‑3p19 and six combined miRNAs (miR-21–5p, miR-23a-3p, miR-30e-5p, miR-221–3p, miR-222–3p, miR-31–5p)31 appeared associated to a better prognosis. In relation to asbestos-related LC, only single miRNAs were associated with diagnosis and/or survival, so we were unable to perform a meta-analysis among the most frequently reported. Of note, miR-126, was confirmed also for LC diagnosis, alone,29 and in association with miR-222.22 Also, miR-1254 and miR-574–5p30 were found associated with early-stage NSCLC samples even months before clinical diagnosis, so potentially useful for LC screening programs. We did not find any promising miRNAs in relation to asbestos-related LC survival.
The strength of our systematic review is the comprehensive search strategy including also the so-called grey literature, as also confirmed by the absence of publication bias in the Egger test for the studies included in the meta-analysis. Also, we used a standard tool to assess study quality. Further, we evaluated both diagnosis and prognosis for both asbestos-related LC and MPM. Finally, we managed to quantify the diagnostic performance of the top miRNAs associated with MPM using a meta-analytic approach, so quantifying their potential role as cancer biomarkers.
We acknowledge several limitations. Almost all studies were case-controls, so subject to reverse causation bias. Almost all studies included men only, and had small sample size, so preventing generalizability of the findings to women. Also, asbestos exposure was usually self-reported and without information on type and duration of exposure, so vulnerable to exposure misclassification; there is even no evidence this was a differential between cases and hospital controls. The studies were also heterogeneous in methodology, and this allowed us to perform a meta-analysis only on a small selected sub-sample of more comparable studies. In addition, given that we pooled AUCs estimated also from multivariable models or studies matched by design, we cannot rule out a certain degree of overestimation.
Several knowledge gaps on this topic remain to be addressed: the accuracy of miRNAs to predict asbestos-related LC and MPM in prospective longitudinal studies of healthy ex-asbestos-exposed subjects, especially among women; the exposure-response relationship between asbestos and miRNA changes; the underlying biological mechanisms, and the influence of internal (e.g. age, sex, genes) and external (e.g. smoking, co-exposure to other occupational agents, therapies) factors on miRNA expression perturbations. Future research addressing these shortcomings in large prospective studies is warranted to shed some light on these issues.
ConclusionTo conclude, in this comprehensive systematic review and meta-analysis we identified some promising miRNA candidates to predict diagnosis and survival of asbestos-related LC and MPM. Future large longitudinal standardized validation studies are needed to confirm these findings, assess their clinical relevance, and address present knowledge gaps. The current poor survival and quality of life for patients affected by asbestos-related lung cancers, especially MPM, urge the identification of accurate and reliable, non-invasive, early diagnostic biomarkers to be included in cancer screening protocols among ex asbestos-exposed subjects, as well as to provide molecular targets for new therapies. However, the best prevention remains to ban asbestos globally to avoid the associated important death toll for the long-term future.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Regione Autonoma della Sardegna, LR 7/2007, “Promozione della Ricerca Scientifica e dell'Innovazione Tecnologica in Sardegna” year 2020; POR project FESR 2014-2020.