Lung cancer is the leading cause of cancer deaths in the entire world, representing 19.4%–27% of all deaths from cancer.1 Surgical resection is considered the treatment standard for early stage disease (stage I and II) and for some cases in IIIA stage.1 Accordingly to the TNM system, 5-years survival for IA, IB, IIA, IIB, IIIA, IIIB and IV stage disease, is about 73%, 58%, 46%, 36%, 24%, 9% and 13% respectively.2
The purpose of this study is to describe a population of patients submitted to curative intent surgery for lung cancer and analyze the survival.
Data was collected retrospectively from the clinical process of patients with NSCLC who had indergone pulmonary resection surgery with curative intent who were being followed at the Pneumological Oncology Service of a University Hospital. Patients who had not had lymphadenectomy were excluded.
After initial treatment was completed, patients were followed every 3 months with a complete physical examination, blood analyses and chest X-ray for 5 years, every 6 months with computed tomography (CT) for 2 years and then annually.
A descriptive analysis of the variables of 102 patients undergoing pulmonary resection surgery was carried out. This included patients who were operated on between 1 January 2008 and 31 December 2012 and were followed until 31 December 2015.
The comparison of the distribution of variables was made using adjustment tests (Binomial and Chi-square). Kaplan–Meier survival analysis was used to determine mean survival time and mortality rate according to the disease stage and comparison of these by log-rank test. The association between pathologic stage and survival was assessed using Fisher's exact test. The analysis was performed in the SPSS, version 23, and the statistical tests analyzed at the significance level of 5%.
The study was conducted with a group of 102 patients with an average age of 63.69±9.31; 68.6% were men. The distribution of tumor location is preferentially peripheral (72.5%), appearing in the lobar bronchus (15.7%) or main bronchus (11.8%) with less frequency (p<0.001). Most of the patients had Adenocarcinomas (61.8%) or squamous tumors (26.5%). Most of the surgeries performed were lobectomies (81.4%), followed by pneumonectomy (14.7%), wedge excision (2.9%) and segmentectomy (1%). 26.5% cases were in pathological stage IA, 24.5% in IB, 21.6% in IIA, 9.8% in IIB and 17.6% in IIIA (p=0.064).
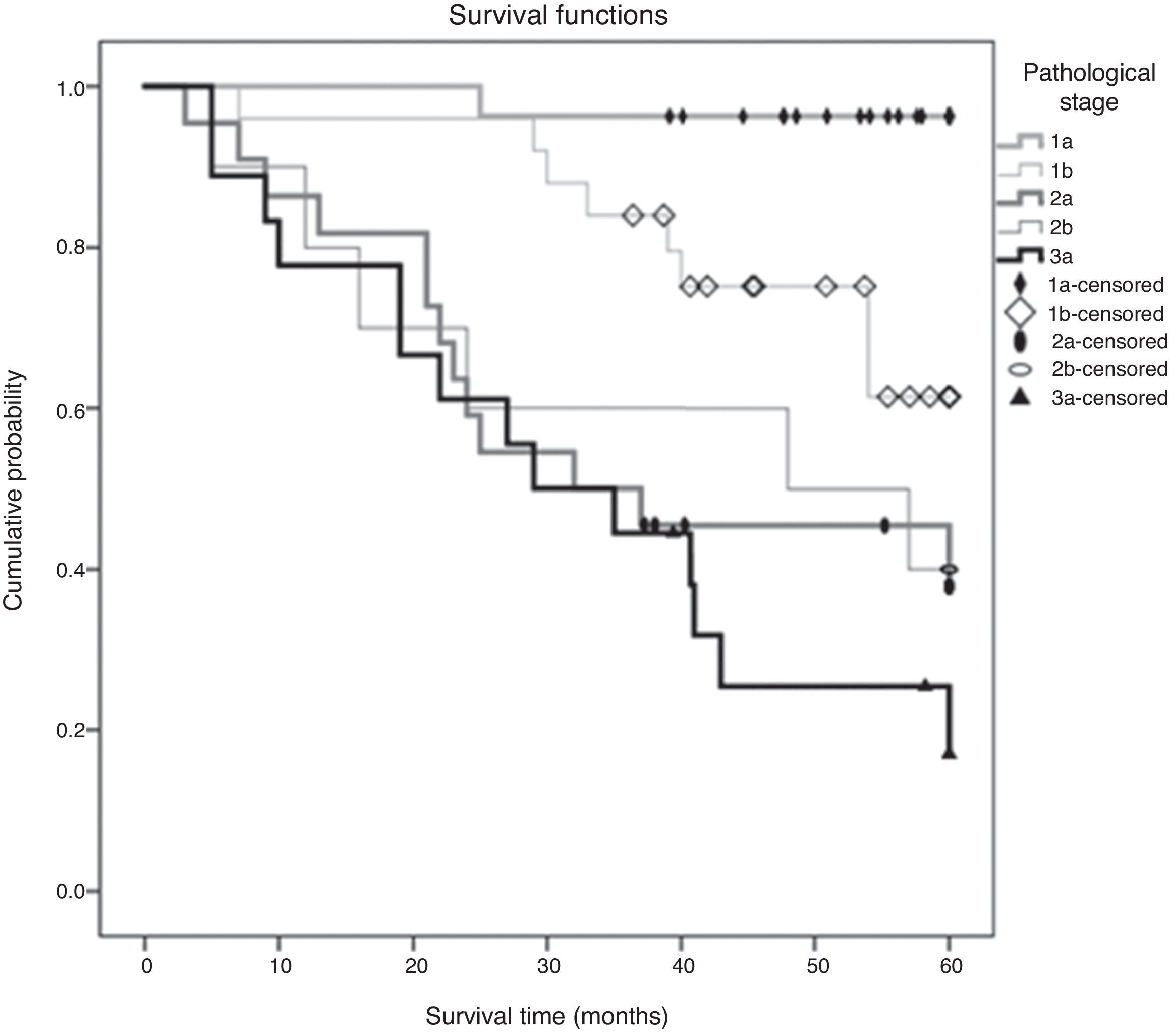
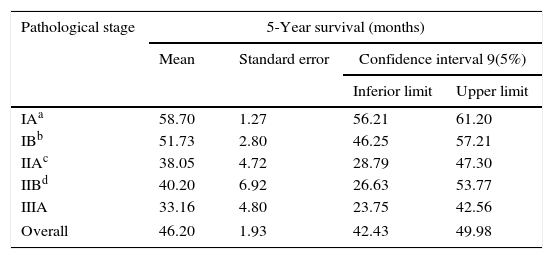
Of the 102 cases analyzed, 41 died during the study period (40.2%). The estimated mortality rate in the population should be between 30.7% and 47.7%. The median overall survival time was 65.61±3.56 months and the 5-year survival (60 months) was 46.20±1.93 months. There was a statistically significant difference in mean survival time depending on the disease stage (p<0.001) [Fig. 1]. The tendency is that patients with stage IA have a better prognosis, with a statistically significant difference between this and any of the other stages. Table 1 presents the mean survival time observed at the 5-year follow-up, and the respective estimated mean survival time in the population, in relation to the disease stage. In the legend, the p-values adjusted for multiple comparisons of the survival time between pairs of stages evaluated are presented.
5-Year survival (months) by stage.
| Pathological stage | 5-Year survival (months) | |||
|---|---|---|---|---|
| Mean | Standard error | Confidence interval 9(5%) | ||
| Inferior limit | Upper limit | |||
| IAa | 58.70 | 1.27 | 56.21 | 61.20 |
| IBb | 51.73 | 2.80 | 46.25 | 57.21 |
| IIAc | 38.05 | 4.72 | 28.79 | 47.30 |
| IIBd | 40.20 | 6.92 | 26.63 | 53.77 |
| IIIA | 33.16 | 4.80 | 23.75 | 42.56 |
| Overall | 46.20 | 1.93 | 42.43 | 49.98 |
Survival time was significantly longer than in the IB stages (p=0.006), IIA (p<0.001), IIB (p<0.001) or IIIA (p<0.001).
Survival time significantly longer than stages IIA (p=0.031) or IIIA (p=0.002), and similar survival time at IIB (p=0.166).
Adenocarcinoma was the predominant histologic type, as in the international literature. (61.8%).3 As expected, the majority of the tumors had a peripheral location, probably related to the predominance of adenocarcinomas.
The majority of our patients were treated with lobectomy plus systematic lymph node dissection, the standard treatment for lung cancer resections with curative intent.1
Our overall survival is much better (65.61±3.56 months) than the 50 months found by Yue et al.4 Our results are similar to those found by Liu et al.5 for stage IA (89.9 versus 88.11 months) and much lower for stage IB (75.5 versus 87.07 months). Although the overall survival in months was encouraging compared to other studies, we found a 5-year survival rate of 58.5%, a lower value compared to the 64.1% found by Qiu et al.6 These different results can be explained by the different inclusion and exclusion criteria used in the different studies.
We found a positive correlation between disease stage and 5-year survival that was 96.3%, 84.0%, 50%, 50% and 25.4% in stages IA, IB, IIA, IIB and IIIA respectively, slightly better results in than those reported by Dziedzic et al.2
Our study has some limitations that should be mentioned. We did not collect data about the number of lymph nodes and which stations were excised or which treatment was performed on each patient, two risk factors that we know can predict mortality.
However, this is the first study to evaluate survival in our country and despite the retrospective nature of this study it is a real life study that gives us information about our population.
Conflicts of interestThe authors have no conflicts of interest to declare.