The mortality associated with high-risk pulmonary embolism (PE) is remarkably high, and reperfusion to unload right ventricle should be a priority. However, several registries report reperfusion underuse. In Portugal, epidemiological data about the incidence, rate of reperfusion and mortality of high-risk PE are not known.
MethodsNationwide population-based temporal trend study in the incidence and outcome of high-risk PE, who were admitted to hospitals of the National Health Service in Portugal between 2010 and 2018. High-risk PE was defined as patients with PE who developed cardiogenic shock or cardiac arrest. International Classification of Diseases (ICD), 9th and 10th revision, Clinical Modification codes, were used for data from the period between 2010 and 2016 (ICD-9-CM) and 2017–2018 (ICD-10-CM), respectively. The assessment focused on trends in the use of reperfusion treatment, which was defined by application of thrombolysis or pulmonary embolectomy. A comparison was made between the use or non-use of reperfusion therapy in order to examine trends in in-hospital mortality among high-risk PE cases.
ResultsFrom 2010 and 2018, there were 40.311 hospitalization episodes for PE in adult patients at hospitals of the National Health Service in mainland Portugal. There was a significant increase in the annual incidence of PE (41/100.000 inhabitants in 2010 to 46/100.000 in 2018; R2=0.582, p = 0.010). The average annual incidence was 45/100.000 inhabitants/year, with 2,7% of the PE episodes (1104) categorized as high-risk. The mortality rate associated with high-risk PE was high, although it has decreased over the years (74.2% in 2010 to 63.6% in 2018; R2=0.484; p = 0.022). Thrombolytic therapy was underused in high-risk PE, and its usage has not increased in recent years (17.3% in 2010 to 21.1% in 2018, R2=-0.127; p = 0.763). Surgical pulmonary embolectomy was used in 0.27% of cases, and there was no registry of catheter-directed thrombolysis. Patients with high-risk PE undergoing reperfusion therapy had lower in-hospital mortality compared to non-reperfused patients (OR=0.52; IC95% 0.38–0.70).
ConclusionIn Portugal, between 2010 and 2018, very few patients with PE developed high-risk forms of the disease, but the mortality rate among those patients was high. The low reperfusion rate could be associated with high in-hospital mortality and highlights the need to implement advanced therapies, as an alternative to systemic thrombolysis.
Acute pulmonary embolism (PE) is the third leading cause of cardiovascular death following myocardial infarction and stroke. It is one of the most preventable causes of death in hospitalized patients.1 High-risk pulmonary embolism occurs when patients with acute PE experience haemodynamic instability, defined as cardiac arrest or obstructive shock.2 Although a small percentage of patients with acute PE present in shock (3–8%)3, the mortality rate associated with this condition is exceptionally high.4 The early adverse outcome is related to right ventricular (RV) failure due to acute pressure overload when > 30–50% of the pulmonary bed is obstructed by thromboemboli.5 Reducing RV afterload should be a priority in these cases. For this reason, primary reperfusion treatment, in most cases using systemic thrombolysis, is considered the treatment of choice in patients with PE and haemodynamic instability.2 Despite these recommendations, several registries report underuse of reperfusion therapy, with only 16–30% of patients receiving systemic fibrinolysis.1,6-8 Surgical pulmonary embolectomy or percutaneous catheter-directed treatment are alternative reperfusion options in patients who have contraindications to systemic thrombolysis.2
In Portugal, there is limited epidemiological data about PE and its prognostic impact. To date, the only published study has estimated an incidence of PE in 35/100.000 inhabitants/year.9 The incidence of high-risk PE and reperfusion rate are unknown. In this present study, we assessed the national trend in PE mortality between 2010 and 2018, and we evaluated the impact of reperfusion on in-hospital mortality, specifically in high-risk PE cases.
MethodsStudy populationRetrospective cohort study based on the Portuguese Hospital Morbidity Database centrally held by the Central Administration of the Health System (ACSS) which is an administrative registry of hospital admissions that occurred in the National Health Service hospitals in Portugal. Patients included were adults (aged >18 years) hospitalized with acute PE between 2010 and 2018 in mainland Portugal. Diagnoses and procedures are coded according to the International Classification of Diseases (ICD), 9th and 10th revision, Clinical Modification codes, ICD-9-CM for the period between 2010 and 2016 and ICD-10-CM for patients admitted between 2017 and 2018 (see Supplementary Table 1 for details of codes used). Pulmonary embolism was defined by codes ICD-9-CM 415.1, 415.13 or 415.19 and ICD-10-CM I26.09, I26.99, I26.02 or I26.92, which could be present in the episode of hospitalization as a primary or secondary diagnosis. Primary diagnosis of PE was defined as a first-listed diagnosis. We evaluated the fifteen most common first-listed diagnoses when PE was not a primary diagnosis.
The incidence of PE in Portugal was estimated by the number of new PE episodes per year and was expressed as 100.000 inhabitants/year calculated as a nine-year average. Resident population estimates were obtained from Statistics Portugal (INE; www.ine.pt) for the years under study.
Patients were classified as high-risk PE if they were considered to be having cardiac arrest or cardiogenic shock, according to the most recent European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines2. Patients with persistent hypotension, which were not coded as cardiogenic shock, were not included in this analysis.
The incidence proportion of high-risk PE was calculated for each year.
We collected data on advanced therapies for high-risk patients with PE including, intravenous thrombolytic therapy, catheter-based approaches, surgical pulmonary embolectomy, and mechanical circulatory support.
Reperfusion treatment was defined as the use of thrombolytic therapy or pulmonary embolectomy.
As a marker of clinical complexity, we calculated The Charlson Comorbidity Index (CCI)10, which is a method of categorizing comorbidities of patients based on the International Classification of Diseases (ICD) diagnosis codes found in ACSS database. Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient. A score of zero indicates that no comorbidities were found. The higher the score, the more likely the predicted outcome will result in death or higher resource use. We have used the modified CCI resulting in the sum of 17 different categories.11 For a listing of the ICD-9-CM and ICD-10 codes used for each category see reference 12 (Supplementary Table 1).
The study used anonymized public domain data and was approved by local ethic committee.
Outcome definitionsThe primary outcome used in our analysis was all-cause in-hospital death. The secondary outcome was the length of hospital stay (in days). Trends in in-hospital mortality and length of hospitalization between 2010 and 2018, on an annual basis, were assessed in all PE patients and in all high-risk PE patients. In addition, trends in in-hospital death were carried out in different age groups: Elderly (aged ≥ 65 years old) and younger patients (less than 65 years old). We also assessed trends in the rate of systemic thrombolysis among all high-risk PE episodes, including different age groups, as well as the use of other advanced therapies. Trends in mortality and length of hospital stay in high-risk PE were compared between the use or non-use of reperfusion therapy.
We also evaluated the occurrence of intracerebral bleeding in patients undergoing systemic thrombolysis.
Statistical analysisContinuous variables were presented as mean ± standard deviation, and a comparison between variables was performed with an independent t-student test. Categorical variables were presented by numbers (n) and percentages (%) and a comparison between groups was performed using Qui-square test or Fisher´s exact test, when appropriate.
Linear regression analysis was used to assess trends over time. The results are presented as the adjusted R-squared (R2). A linear regression model was parameterized to model the relationship between mortality (dependent variable) and time (years 2010 to 2018).
The time trend analysis of the incidence rate of high-risk PE episodes, mortality rate of high-risk PE, trends in the use of reperfusion therapy, mortality rate of reperfused and non-reperfused high-risk PE were estimated using a joinpoint regression analysis. This method establishes whether significant changes occur overtime and identify timepoint(s) with significant inflections (joinpoint).13
A logistic regression analysis model was estimated to assess in-hospital death among high-risk PE patients, as a function of year, age, gender, Charlson index, primary diagnosis of PE (first-listed diagnosis) and performance of reperfusion procedures. The results are presented as odds ratio (OR) and corresponding 95% CI.
All reported p values were 2-sided and a p value < 0.05 was considered statistically significant.
Statistical analysis was performed with IBM SPSS Statistics 21.0 (IBM Corp, Armonk, NY, USA) and R 4.1.1 (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. The joinpoint regression analysis was performed with the Joinpoint Regression Program, Version 5.0.1. April 2023 (Statistical Research and Applications Branch, National Cancer Institute)
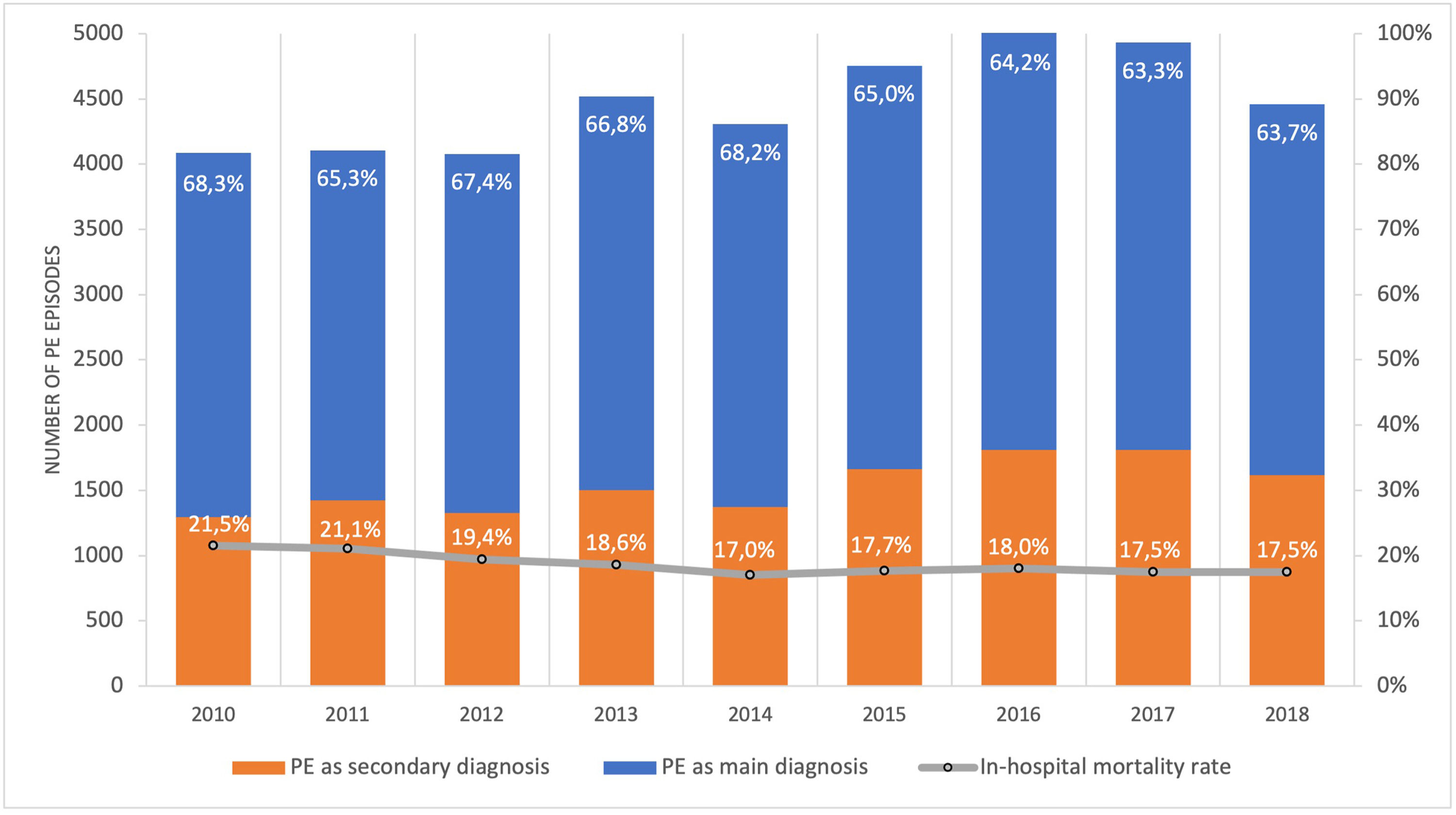
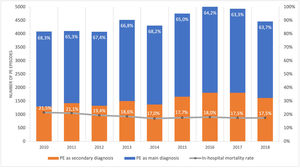
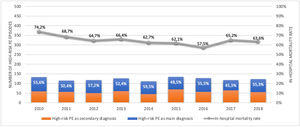
ResultsPopulation demographic study and incidence rate of PE in PortugalBetween 1st January 2010 and 31st December 2018, there were 40.311 episodes of hospitalization for pulmonary embolism in adult patients at hospitals of the National Health Service in mainland Portugal (Fig. 1). PE was the main diagnosis in 65.7% (26.492) of the episodes. This is a disease that mostly affects the elderly (Supplementary Fig. 1 illustrates the age pyramid for this disease). There was a significant increase in the annual incidence of PE (ranged from an annual incidence of 41/100.000 inhabitants in 2010 to 46/100.000 in 2018; R2=0.582, p = 0.010; Supplementary Fig. 2). The average annual incidence was 45/100.000 inhabitants/year. Chest computed tomography (CT) was performed in 69.7% of all PE episodes (ranged from 65% in 2010 and 68% in 2016; R2=0.126; p = 0.230).
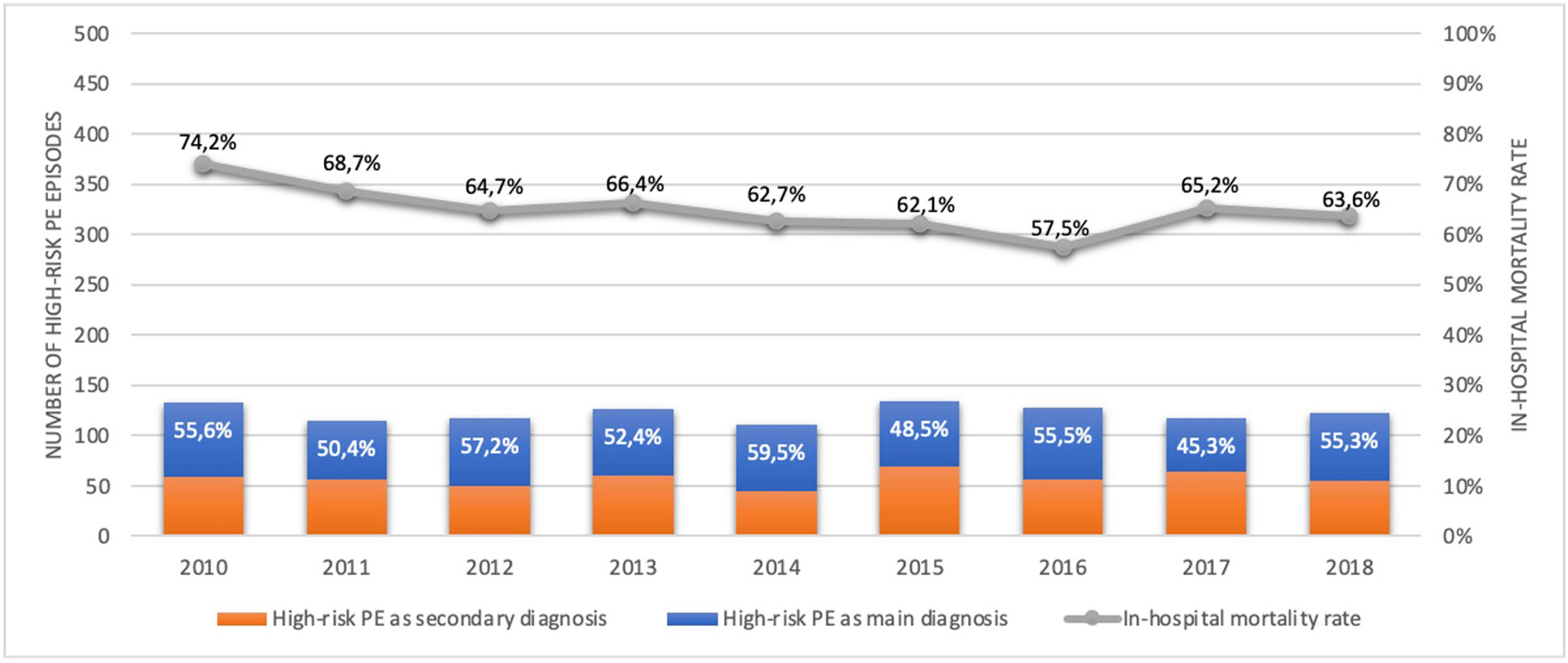
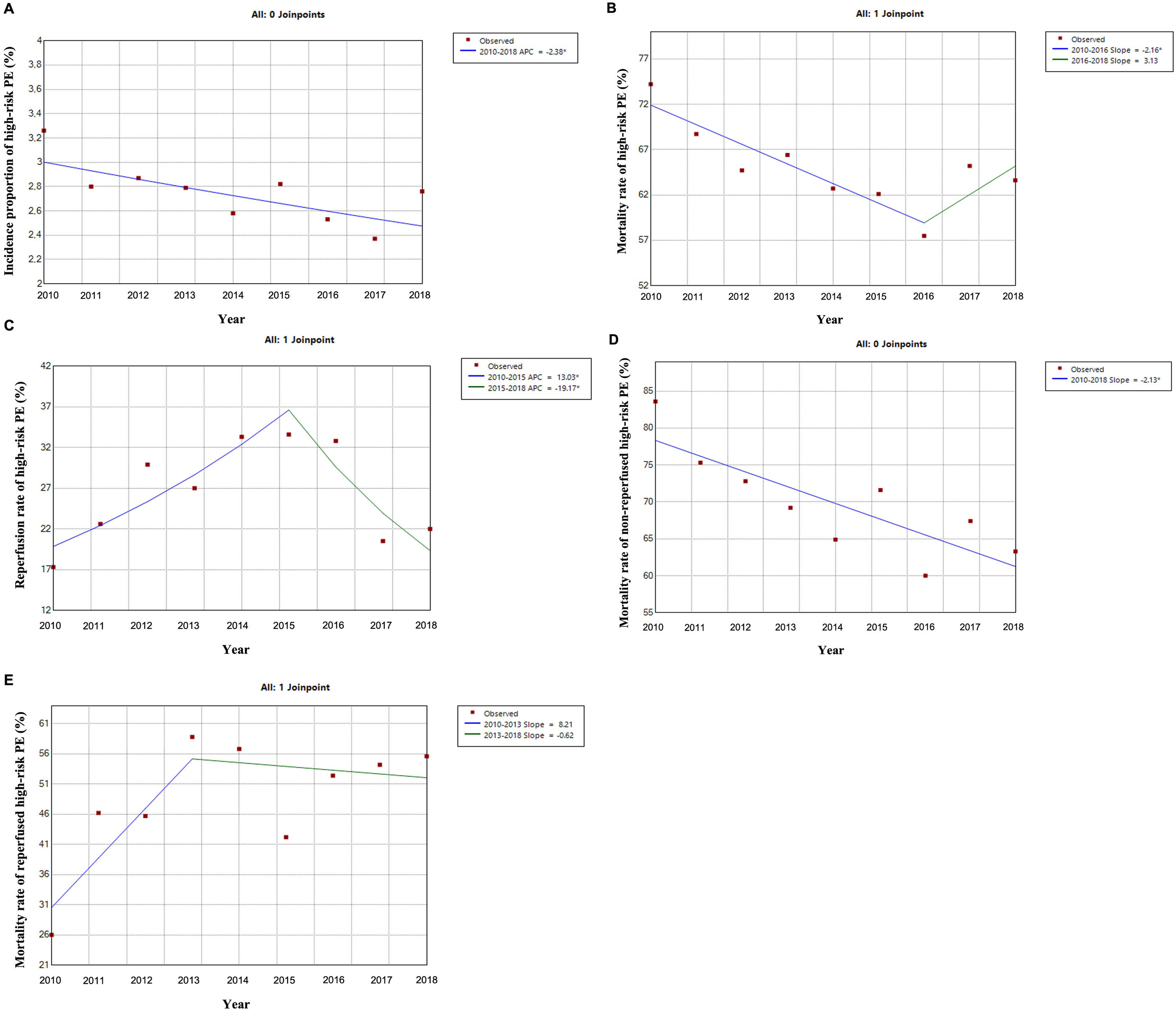
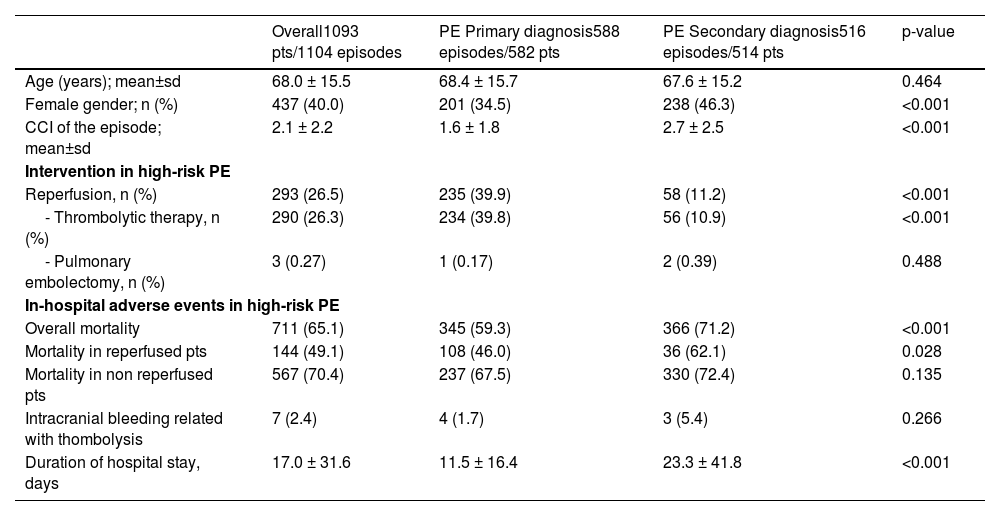
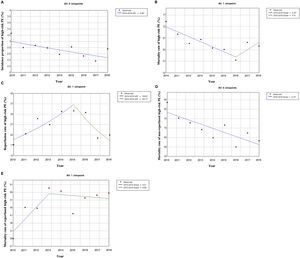
Out of all the PE episodes, 1104 (2.7%) developed high-risk PE with cardiogenic shock or cardiac arrest. The relative percentage of high-risk PE episodes decreased slightly over the years (3.3% in 2010 to 2.8% in 2018; R2=0.422, p = 0.035; Fig. 2) and no joinpoint was identified (Fig. 3A). Chest CT was performed in 52.9% of all high-risk PE and its use increased slightly over the years (46% in 2010 to 57% in 2016; R2=0.648, p = 0.018). Demographic and clinical characteristics in high-risk PE are shown in Table 1. The fifteen most common first-listed diagnoses when PE was not a primary diagnosis are presented in Supplementary Table 2.
Demographic and clinical characteristics in high-risk PE.
CCI: Charlson Comorbidity Index; PE: pulmonary embolism; pts: patients; sd: standard deviation.
There was a slight decrease in in-hospital mortality among all patients admitted with PE over the years (from 21.5% in 2010 to 17.5% in 2018, R2=0.687, p = 0.004; Fig. 1). The mortality in high-risk PE was high, although it has significantly decreased over the years (74.2% in 2010 to 63.6% in 2018; R2=0.484; p = 0.022; Fig. 2). One joinpoint was identified in the year 2016, generating two different linear trends in the mortality rate (Fig. 3B). Mortality in all-risk strata of PE between 2010 and 2018 was, on average, higher in elderly (≥ 65 years) compared with younger patients (20.9% vs 13.7%, p<0.0001). In high-risk PE, mortality of younger patients was high, with fluctuations over the years, although lower than older patients (59.4% vs 68.0%; p = 0.005).
Mortality in overall PE patients decreased throughout the years 2010–2018 both in elderly (R2=0.707; p = 0.003) and young (R2=0.636; p = 0.006). Mortality in high-risk PE did not decrease significantly in neither elderly (R2=0.253; p = 0.096) nor young (R2=0.277; p = 0.084).
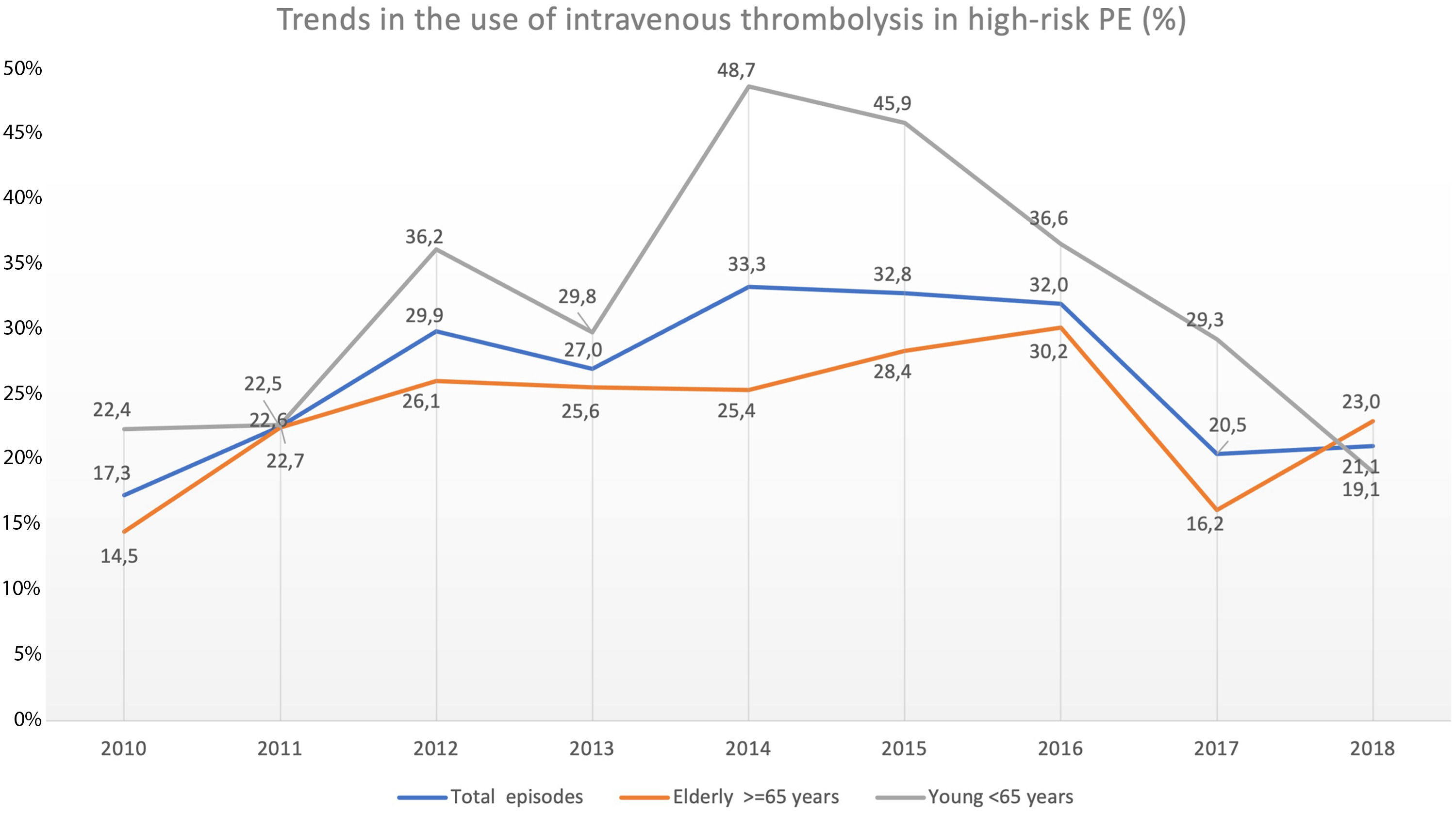
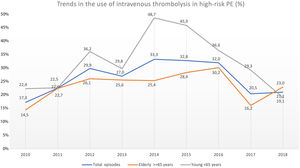
Trend in the use of reperfusion therapy in high-risk PEThrombolytic therapy was underused in high-risk PE, and its use has not increased significantly in recent years (17.3% in 2010 to 21.1% in 2018, R2=−0.127; p = 0.763; Fig. 4).
Trends in the use of intravenous thrombolysis in high-risk PE in overall population, elderly and young, throughout the years 2010–2018. The rate of reperfusion with systemic thrombolysis in patients with high-risk PE was very low and has not increased in recent years (R2=−0.127; p = 0.763).
The use of thrombolysis in high-risk PE was lower in older patients (≥ 65 years) compared to younger patients (23.7% vs 31.6%, p = 0.005). Over the last few years, there have been some fluctuations in the use of systemic thrombolysis in both elderly (14.5% in 2010 to 23.0% in 2018; R2=−0.084, p = 0.556) and younger patients (22.4% in 2010 to 19.1% in 2018; R2=−0.131, p = 0.792) but without any significant increase between 2010 and 2018 (Fig. 4). The use of thrombolysis in the presence of the most important comorbidities is detailed in Supplementary Table 3.
There was 2.4% (7 in 290 episodes) of intracranial bleeding in patients with high-risk PE submitted to thrombolysis and mortality rate in these patients was 57.1% (4 in 7 patients).
The use of advanced therapies as an alternative to systemic thrombolysis in patients with PE was vestigial, with no increase in recent years. Surgical pulmonary embolectomy in high-risk PE was used in 0.27% (3 of 1104 episodes) and mortality was 33.3% (1 of 3 patients). There is no registry of any case of catheter-directed thrombolysis in patients with high-risk PE. Extracorporeal membrane oxygenation in high-risk PE was used in 0.09% (1 of 1104 episodes) and mortality was 100%. Inferior vena cava filter was used in 0.63% (7 of 1104 episodes) and mortality was 71.4% (5 of 7 patients).
The rate of reperfusion (thrombolysis or pulmonary embolectomy) in patients with high-risk PE was very low (26.5%). We identified one joinpoint in the use of reperfusion in the year 2015, generating two different linear trends in the rate of reperfusion: a significant increase in the first years and then a decrease of use in more recent years (Fig. 3C).
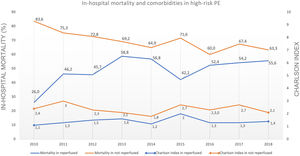
Non-reperfused patients had a higher rate of comorbidities.
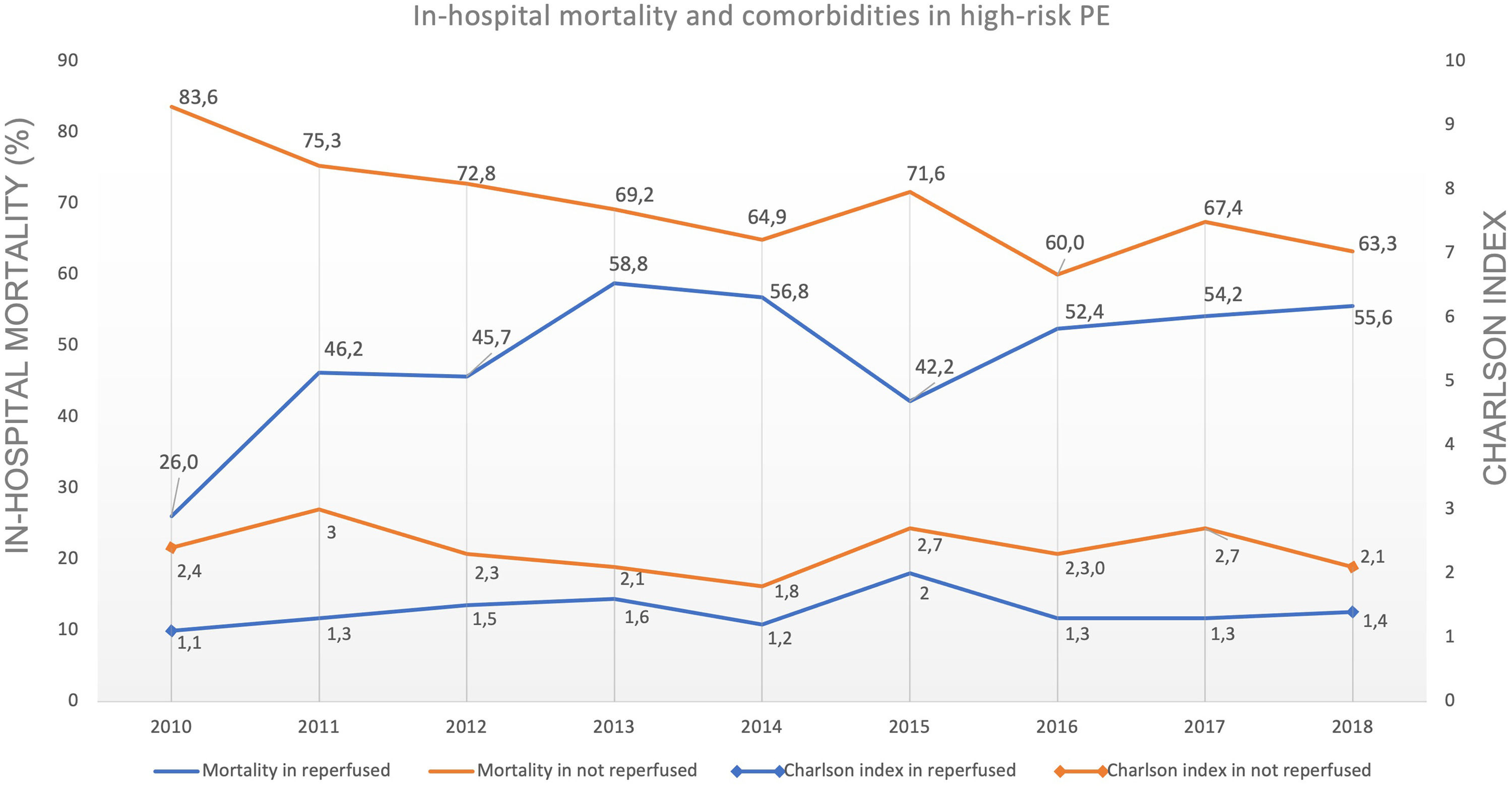
Predictive factors related to hospital mortality in high-risk PEFig. 5 illustrates, over the years of the study, the trends of comorbidities and in-hospital mortality in high-risk PE within reperfused and non reperfused patients. It shows that mortality is higher for patients without reperfusion procedures than among those who underwent reperfusion (in all the years of analysis). There is a linear relationship between death and year for the total population of high-risk PE patients without reperfusion (R2=0.602, p = 0.008) meaning a significant decrease in mortality in these patients over the last few years, with no joinpoint identified (Fig. 3D). In reperfused patients, mortality tended to increase over the years (R2=0.300, p = 0.073). Joinpoint regression analysis identified one joinpoint in the year of 2013 (Fig. 3E). In the following years, the apparent decrease in mortality rates were not significant.
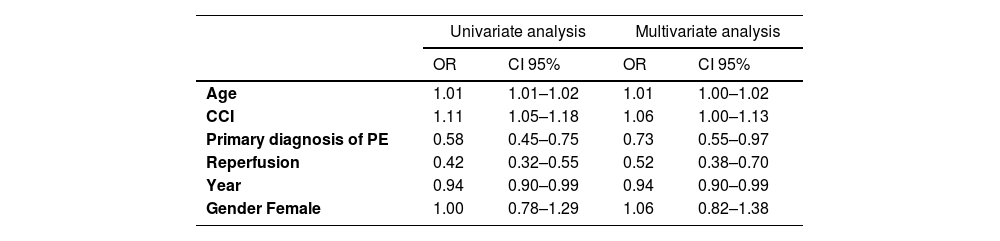
A logistic regression model was estimated to assess predictors of in-hospital death among high-risk PE patients, and gender was considered as a confounding variable, not being statistically significant. Table 2 shows the odds ratio (OR) obtained for each variable, whose coefficient is statistically different from zero (p-value < 0.05), and respective limits of the 95% confidence interval:
Univariate and multivariate analysis for prediction of in-hospital mortality.
CCI: Charlson Comorbidity Index; Variables that entered the model: year of PE hospital admission; age; gender, Charlson Comorbidity Index, primary diagnosis of PE, reperfusion (thrombolysis or pulmonary embolectomy).
Both age and the CCI were associated with a slight increase in the risk of death. Each year of age increases the odds of death by 1%, and a 1-unit increase in the CCI increases the odds of death by 6%, under the same circumstances (fixing the other variables in the model). Patients with PE as a primary diagnosis have 27% less chance of death than those with PE as a secondary diagnosis. Patients who underwent reperfusion procedures are 48% less likely to die when compared to those who did not undergo reperfusion, regardless of whether pulmonary embolism is first-listed or not. The year of the study was also related with the risk of in-hospital death; the more recent the years the lower the risk of in-hospital mortality.
Length of hospital stay in PE patients in PortugalThe average length of hospital stay in the total episodes of PE has declined over the years (15.9 days in 2010 to 14.1 days in 2018, R2=0.804, p<0.001). The length of hospital stay in severe forms of PE episodes was high (average of 17.0 days), and has not changed significantly in recent years (17.2 days in 2010 to 16.4 days in 2018, R2=0.050, p = 0.273). Patients with high-risk PE undergoing reperfusion had similar hospital stays compared to non-reperfused patients (the average in the period of 2010–2018 was 16.0 ± 5.0 days in reperfused patients vs 17.4 ± 2.5 in non-reperfused; p = 0.345).
DiscussionThe main findings of this retrospective cohort study of patients hospitalized due to PE in the mainland Portugal in the last decade (2010–2018) were as follows: 1) The average annual incidence was 45/100.000 inhabitants/year between 2010 and 2018; 2) of all episodes of PE, 2.7% developed high-risk PE with cardiogenic shock or cardiac arrest; 3) The mortality in high-risk PE was high, although it has decreased over the years; 4) reperfusion rate with systemic thrombolysis as a first-line therapy was underused in high-risk PE, and its use has not increased in recent years; 5) use of alternative reperfusion methods to systemic thrombolysis in PE until 2018 was negligible; 6) short-term survival was higher in PE patients with shock or cardiac arrest who underwent reperfusion.
In Portugal, epidemiological data about the incidence, the rate of reperfusion and mortality in high-risk PE are not known. The only nationwide study available reporting the numbers of PE in Portugal is from Gouveia M. et al.9, that estimated a PE incidence of 35/100.000 inhabitants and an overall in-hospital mortality rate of 17% in 2013. In our study, with more recent data, the average annual incidence was 45/100.000 inhabitants/year and in-hospital mortality rate decreased slightly over the years (from 21.5% in 2010 to 17.5% in 2018). Gouveia M. et al.9 explains this decrease in mortality, in part, by a greater ability to diagnose less severe forms of PE due to the increasing use of CT scans. According to the author, what most contributed to the reduction of mortality has been an improvement in hospital health care effectiveness.
The proportion of patients with PE stratified as high-risk in Portugal in the last decade (2010–2018) is estimated at 2.7%, in accordance with previously reported numbers of other nationwide studies, of whom a small proportion of patients with PE presented with shock (<5%).3,14,15 In the National Inpatient Sample with data of PE hospitalizations, from the period of 1999–2014, in all regions of the United States, high-risk patients were 4,8%.16 In the International Cooperative Pulmonary Embolism Registry (ICOPER), with data of 2454 consecutive PE patients between 1995 and 1996 in 52 European and North American hospitals, 4.2% had massive PE (103 patients), defined as a systolic arterial pressure <90 mm Hg.1 In the international prospective Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) registry, between 2001 and 2006, 15.520 consecutive patients with acute venous thromboembolism were included, and symptomatic massive PE was present in 3.8% (248 of 6512 patients with PE).14 In The Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry (EMPEROR),15 the rate of high-risk PE was lower (3.1%) and more in line with those found in our national data. Contrary to the increase in the incidence of pulmonary embolism in recent years due to the aging of the population and the increase in diagnostic capacity, the incidence of high-risk forms in our study has decreased slightly over the years. The reasons that explain the increase in the incidence of PE at a global level (aging of the population, wide availability of CT scan, a greater awareness of PE among clinicians and the increase in the incidental diagnosis of PE)17,18 are not reasons that could contribute to the increase in the incidence of the highest risk forms of PE. On the other hand, the increased awareness of PE and the greater adoption of diagnostic algorithms could even lead to an earlier diagnosis of PE, a faster initiation of anticoagulant therapy and reduced progression to haemodynamic decompensation. This could be an explanation for the low numbers found of PE episodes with cardiogenic shock or cardiac arrest in recent years, although the authors cannot exclude underreporting or miscoding with data analysis based on ICD discharge codes. Indeed, the authors cannot exclude the possibility that there might be an underdiagnosis of the most severe forms of PE, as cardiac arrest and sudden death may occur before the diagnosis of PE. This is supported by previous autopsy studies.19,20
Contrary to what happened in most PE patients, the mortality rate of critically ill patients with high-risk PE was high in recent years (74.2% in 2010 to 63.6% in 2018). German registry7 also published very similar mortality rate in hemodynamically unstable PE patients (76.6%). However, it remains higher than what was found in previous registries, where the mortality rate in PE with shock or cardiac arrest was usually close to 50%: in-hospital all-cause mortality rate of 52.2% for high-risk PE in the Nation Inpatient Sample (NIS) from United States16, 58.3% of mortality at 3-months in high-risk PE in ICOPER Registry1. One of the reasons that may explain the lower mortality in the United States NIS registry16 is that they only included patients with PE as the main diagnosis (first-listed). In our registry, the overall mortality was 65.1%, but if we analyze the subgroup of patients with first-listed PE, the mortality was lower (59.3% compared to 71.2% in patients whose pulmonary embolism was a secondary diagnosis) and closer to other previous registries. Nevertheless, this high mortality is concerning and may reflect the low rates of reperfusion by systemic fibrinolysis or other advanced therapies in this subgroup of patients. Indeed, this study is the first large-scale nationwide study in Portugal that suggests a correlation between the use of reperfusion and a lower risk of in-hospital mortality. In multivariate analysis, patients who undergo reperfusion procedures are 48% less likely to die compared to those who did not undergo reperfusion. However, upon analyzing the data from Figs. 3 and 5, it showed that in reperfused patients, mortality tended to increase in the first years (until 2013), in contrast to non-reperfused patients. This increase of mortality among reperfused patients is not due to the increase in comorbidities in the analysed population, as indicated by the Charlson score. We can hypothesize that the increase in mortality was related to the greater use of thrombolysis in an older population, as age is another independent predictor of mortality according to our logistic regression model. If we offer reperfusion to a broader population with clinical indication, we may be including patients for whom the intervention is futile.
Another potential clinical benefit of reperfusion is the reduction in the length of hospital stay.21 This may be true in normotensive patients with intermediate-risk PE when the use of thrombolysis was compared with isolated anticoagulation,21 but this finding was not reproduced in our highest risk population. The length of hospitalization in high-risk PE could be influenced by the potential clinical benefit of reperfusion, as well as complications associated to it. Furthermore, main diagnoses when PE is a secondary diagnosis and associated comorbidities will certainly affect the length of hospital stay.
The rate of use of systemic thrombolysis in Portugal is very low (21.1% in 2018), considering that this is a first-line therapy in patients with high-risk PE.2 Although the authors cannot completely exclude the possibility of miscoding and underreporting the use of thrombolysis due to the nature of the analysis based on ICD codification, these low numbers are in agreement with other registries published in the literature. In the multicentric ICOPER1 registry two thirds of patients were ineligible for fibrinolysis; in RIETE6 only 20% of HD unstable patients were reperfused; in a German registry7 of 885.806 PE patients, systemic thrombolysis was used in only 23% of HD unstable patients; in a nationwide registry from United States, with 58.784 patients hospitalized with high-risk PE, from 1999 to 2017, thrombolytic therapy was administered to 16.1% of patients, open pulmonary embolectomy alone in 4.3% of cases and extracorporeal membrane oxygenation in 0.4% of cases.8 As expected, non reperfused patients had higher CCI, because patients with worse baseline clinical conditions often have absolute or relative contraindications to the use of systemic thrombolysis (for example, cerebrovascular disease, advanced liver disease or tumor with active bleeding).22 In terms of age, elderly patients were numerically reperfused less frequently compared to younger patients. Although, elderly patients with acute PE have higher mortality risk and could benefit more from reperfusion therapy, concerns about bleeding complications with thrombolysis in the elderly, could be an explanation for underuse of this therapy.23
One of the fears associated with using systemic thrombolysis is the risk of intracranial hemorrhage, which occurred in 2.4% of cases in our study, similar to the 2.0% rate of hemorrhagic stroke that occurred in the tenecteplase group of PEITHO trial.24 This fear of hemorrhagic complications could be an explanation for the joinpoint finding in 2015 about the use of reperfusion in our high-risk PE population. After the publication of PEITHO trial in 2014,24 clinicians may have increased their fears of using systemic thrombolysis, even in patients with cardiogenic shock. This reduction in the use of thrombolysis after 2015 was more evident in younger patients, which is concerning.
In Portugal, the use of advanced therapies as an alternative to systemic thrombolysis in patients with PE was vestigial, with no increase in recent years. This data is also worrying, as the guidelines published by ESC/ERS recommend surgical pulmonary embolectomy or catheter-directed therapies (CDT) as class IA and IIA (level of evidence C), respectively, in high-risk PE for patients in whom systemic thrombolysis has failed or is contraindicated, taking into consideration the local experience and available resources.2 In fact, to improve the survival rate in most severe forms of PE, emergent embolectomy surgery or percutaneous catheter-directed treatment (CDT) could be effective alternatives that broaden the spectrum of patients who can undergo reperfusion. However, few patients in Portugal have access to advanced therapies, as an alternative to systemic thrombolysis. This is often a result of a lack of awareness, insufficient funds and resources, and a lack of coordination between all stakeholders involved. The establishment of a healthcare system for high-risk PE patients, needs to be developed at a national level.25 Pulmonary Embolism Response Teams (PERT) should be established, as multidisciplinary and rapid response teams, that would allow rapid signaling of patients at higher risk, as well as fast reperfusion treatment delivery.2,26 Access to PERT leads to the mortality reduction in severe forms of PE.27 In addition to creating PERT, it is necessary to organize a PE response network in order to always offer the best therapy based on the patients geographical location and according to local resources, being able to offer a 24 hours a day, 7 days a week (24/7) service with nationwide coverage.25
Despite the high mortality found in high-risk PE, it decreased significantly from 2010 to 2018, and linearly in non reperfused patients, suggesting an improvement in the treatment of patients in cardiogenic shock or cardiac arrest, rather than a reflection of greater use of advanced reperfusion therapy.
Study strengths and limitationsOur study´s strength is the power afforded by the Central Administration of the Health System´s (ACSS) database, which include access to millions of inpatients with acute PE from all the hospitals of the National Health Service in mainland Portugal. Data obtained from the recent nine years (2010 to 2018) allows for a robust study of national trends in the incidence and outcomes of high-risk PE.
One of the study´s limitation was that the population of Madeira and Azores islands, as well as information regarding inpatients from private hospitals, and acute PE patients treated as outpatients, were not included in this analysis, which could underestimate the incidence of PE in Portugal. The second limitation was the retrospective nature of the study, dependent on administrative data based on ICD coded information, as it relies on the accuracy of ICD-9-CM and ICD-10-CM codes. Coding practices were developed for reimbursement and not for clinical issues. However, previous studies have demonstrated a good level of accuracy of these codes with confirmed acute PE cases.28,29 Thirdly, we have selected the patients with PE as first or secondary code, which may lead to false positives diagnosis, as primary diagnosis codes from hospitals were more likely to represent acute PE than secondary diagnosis codes.30 However, it is known that hospitalization for other clinical reasons (in elderly people and those with cancer, congestive heart failure, chronic pulmonary disease or undergoing major surgery) is a strong or moderate risk factor for PE31. For that reason, we would be underestimating the incidence of PE in Portugal if we did not include PE as a secondary diagnosis. Another limitation is related with the definition used in this study to classify high-risk PE. Patients were classified as high-risk PE if they were considered as having cardiac arrest or cardiogenic shock, but we could have missed some patients with persistent hypotension without obstructive shock who, according to the most recent European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines2 are also considered high-risk PE patients. Thus, we may have underestimated the incidence of high-risk PE in Portugal, and we may have overestimated its mortality by selecting the most severe patients. On the other hand, as the study was based on administrative data based on ICD coded information there could be an underestimation of medical procedures performed, such as, thrombolysis and chest CT. Finally, the outcome of “all-cause in-hospital mortality” may worsen the prognosis of pulmonary embolism in the selected patients in which PE was not the primary diagnosis. In these cases, PE-related death could be a more desirable outcome, but harder to measure in a retrospective study.
ConclusionsIn Portugal, although the incidence of pulmonary embolism has increased in recent years, very few patients with pulmonary embolism have developed high-risk forms of the disease. However, systemic thrombolysis was underused, and in-hospital mortality rate of those high-risk PE patients was very high. Low reperfusion rate could be associated with high in-hospital mortality and applying advanced therapies, as an alternative to systemic thrombolysis, should be implemented.
This research received no financial support.