A 57-year old woman underwent lung transplantation for non-specific interstitial pneumonia. Primary graft dysfunction was diagnosed requiring continued use of extracorporeal membrane oxygenation (ECMO). Within three days she developed recurring hemothoraces requiring two surgical evacuations. After ECMO removal a series of complications occurred within four months: femoral thrombosis, persisting tachycardic atrial fibrillation, pneumopericardium with an esophagopericardial fistula and purulent pericarditis, septic shock, multiorgan failure and intracerebral hemorrhage with ventricular involvement requiring external ventricular drainage. Interdisciplinary management coordinated by the intensive care specialist, transplant surgeon and pulmonologist with various interventions by the respective specialists followed by intensive physical rehabilitation allowed for discharge home on day 235 post transplant. Subsequently quality of life was considered good by the patient and family.
Lung transplantation (LTx) is a standard procedure for selected end-stage lung diseases. Complications such as infection, rejection and malignancy contribute to the inferior survival rates of lung transplant recipients (LTR) as compared to other solid organs transplant patients. Due to polymedication, complications may present and respond to treatment differently and serial complications may be challenging for patients, relatives and healthcare professionals. We communicate here our most challenging case encountered in two decades of LTx.
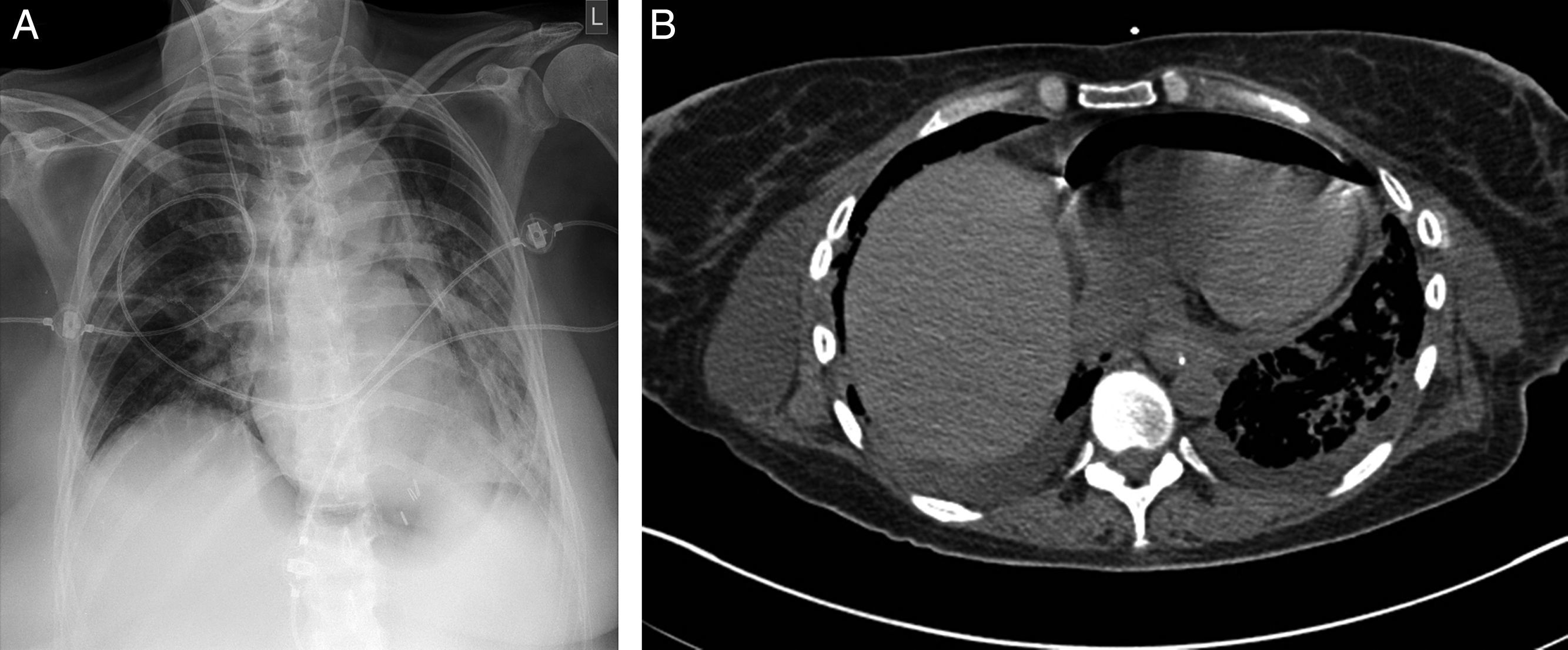
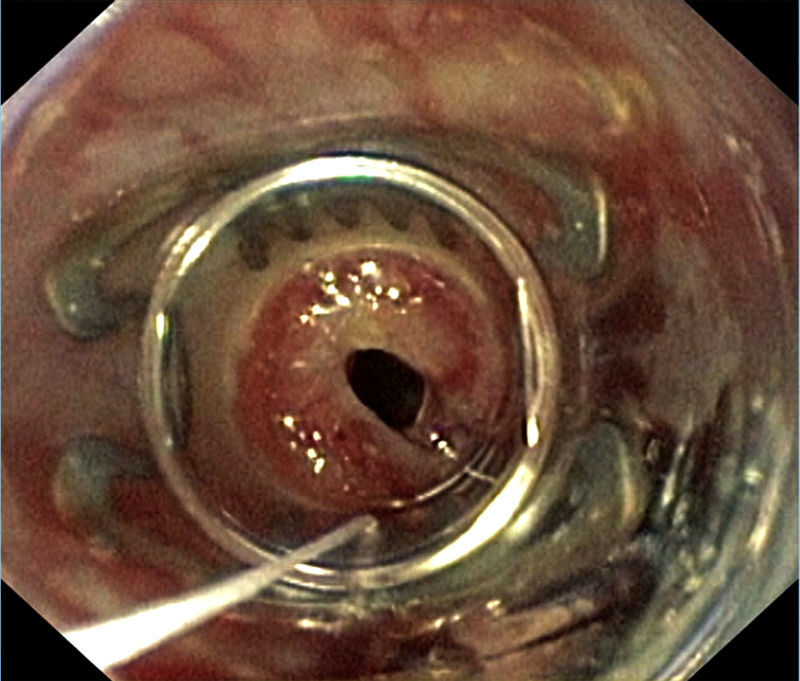
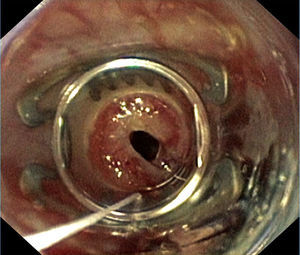
Case reportA 57-year old woman received bilateral LTx for advanced non-specific interstitial pneumonia diagnosed by biopsy prior to transplantation. Her previous medical history was notable for a pulmonary sarcoidosis diagnosed 10 years ago and pandiverticulosis without infection. Mild pulmonary hypertension (PH) pre-transplant of 30mmHg was known. Intraoperative clamping of pulmonary artery led to a mean pressure of 40mmHg, so that ECMO was installed. In patients with PH requiring ECMO for transplant we routinely continue ECMO for weaning in the ICU setting. Postoperatively she remained ECMO-dependent due to a primary graft dysfunction. Within 24h postoperatively she developed a left-sided hemothorax that needed surgical evacuation. The left atrium anastomosis was the source of the bleeding. Two days later a recurrence of the hemothorax occurred requiring surgical revision. These bleedings were facilitated by reduced coagulation due to heparin use required for ECMO. The improved respiratory situation allowed for removal of veno-arterial ECMO immediately after the second hematoma evacuation. A tracheostomy was performed due to expected extended ventilation requirements. An enteral feeding tube was installed endoscopically on postoperative day 4 in order to provide postpyloric feeding and medication. Gastroscopy showed some gastric erosions likely due to the nasogastric tube. On day 7 swelling of the right leg led to the diagnosis of thrombotic occlusion of the external iliac, common femoral and proximal femoral veins by duplex sonography requiring therapeutic anticoagulation. Thrombosis was considered a likely consequence of large-bore cannulation for ECMO.1 A brief bronchoscopic evaluation for unexplained increased C-reactive protein of 76mg/L on day 9 showed no evidence for anastomotic or infectious complications. For persisting atrial fibrillation amiodarone was started. Subfebrile temperatures were noted on day 11–15 with no obvious focus of infection from extensive sampling. Only recurrent gastroesophgeal reflux was detected, so antireflux measures were increased. Successful weaning led to decannulation on day 22 and transfer to the regular transplant ward two days later. On day 26 dyspnea, palpitations and anxiety revealed recurrent tachycardic atrial fibrillation requiring intermediate care admission for additional IV amiodarone loading doses. After a central venous line replacement the chest x-ray showed a pneumopericardium (Fig. 1A), which was confirmed by chest CT scan (Fig. 1B). Within 4h the patient deteriorated rapidly due to septic shock leading to reintubation, circulatory support, hemofiltration and escalation of the antimicrobial treatment. Diagnostic evaluation revealed signs of reflux and candida esophagitis, esophagopericardial fistula with a visible 6mm opening in the posterior left aspect of the esophagus requiring placement of a partially covered 12cm self-expandable esophageal stent. A percutaneous pericardial tube drainage/flush system was inserted showing purulent pericardial effusion and retracheostomy was performed. Enterococcus faecium was cultured in the endobronchial wash from the apical left lower lobe infiltrate and the same organism was repeatedly cultured from the pericardial effusion during the first 5 days of the 3 week drainage period. Intravenous teicoplanin and caspofungin were given and daily pericardial taurolidine instillations were performed. Stent and tracheostomy removal were possible on day 43 and 48 post-transplantation respectively. Visible persistence of the fistula orifice in the esophagus required immediate closure with an Over-The-Scope-Clip (Fig. 2). Transfer from ICU to the regular transplant ward was possible on day 56. Intermittent dialysis was still required until day 63. Due to critical illness polyneuropathy/myopathy intensive inpatient rehabilitation was necessary.
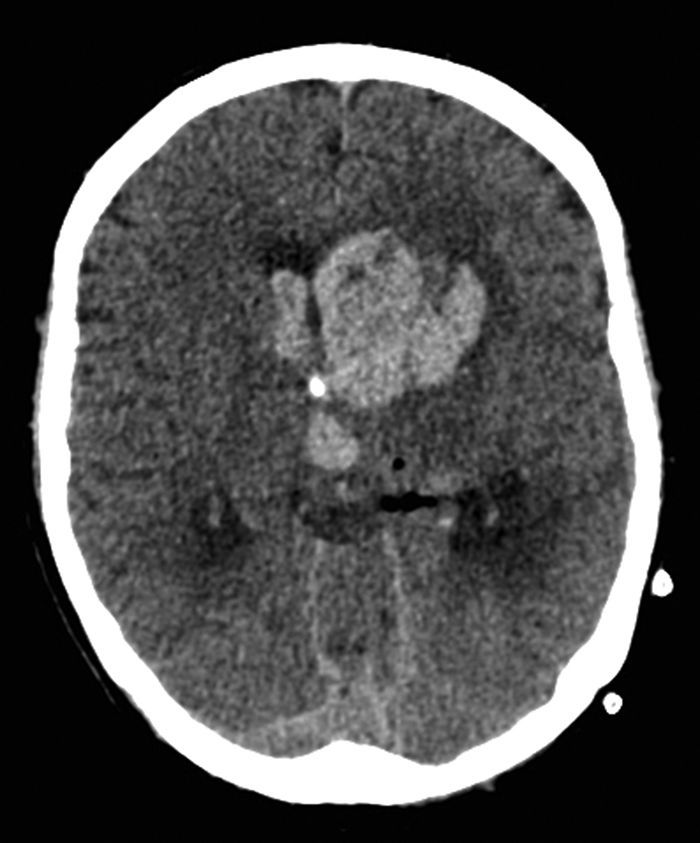
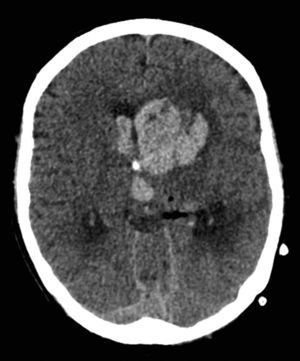
On day 107 the patient showed no arousal and response to physical stimulation. The CT scan of the brain revealed a bleeding in the basal ganglia of the left side that had ruptured into the ventricular system with subsequent occlusive hydrocephalus (Fig. 3). This complication occurred on correctly dosed and monitored therapeutic IV heparin. Previously an attempt to change to oral anticoagulants had failed due to strongly variable dosing requirements. Despite immediate bilateral insertion of external ventricular drains the patient remained comatose. The EEG showed epileptic potentials so that anti-seizure medication was initiated which lead to partial reversal of the comatose state within hours. Right-sided hemiparesis persisted after ventricular drain removal 18 days later. Therapeutic anticoagulation was not reintroduced based on this serious complication. Prophylactic heparin was given after the 20 day ICU stay for this complication. Due to strong dependency on help of the nursing staff and the intensive therapies by physiotherapists and ergotherapists the transfer to a rehabilitation clinic was only possible on day 158 after progress in mobility and self-care.
After additional 11 weeks of specialized neuro-rehabilitation discharge home was possible with help of her working husband and two daughters living nearby. No additional help was needed despite some persistent cognitive deficits and moderately decreased motor function allowing for mobility with a rolling walker. The patient was able to climb one flight of stairs alone using a handrail. Physiotherapy was continued as an outpatient. Eleven days later she was rehospitalized due to double vision with a suspected right-sided internuclear ophthalmoparesis. Two days after exclusion of cerebral bleed in cranial contrasted MRI and CT scans, the occulomotorius paresis was less pronounced permitting discharge on day 3. Lung function subsequently improved to the best average baseline forced expiratory volume in one second of 1470ml, 61% of predicted on day 308. By this time the patient was self-sufficient and came to outpatient visits by public transport on her own. Her quality of life as judged by the patient and family was considered good.
Twenty-seven months postransplant she was readmitted with progressive dyspnea due to segmental pulmonary emboli. Subsequent transient deteriorations in lung functions were seen mainly due to viral respiratory tract infections, which correlated with the frequent contact with her grandchildren experiencing respiratory infections. No additional hospitalizations were required. After the pulmonary emboli the spirometric results deteriorated despite anticoagulation to an average of 500ml two months before she died on day 1027 at home of progressive respiratory failure requiring supplemental oxygen for the last weeks of her life. Autopsy showed advanced vascular rejection, pathoanatomical evidence of pulmonary hypertension with right-sided cardiac hypertrophy and bilaterally dilated ventricles. No signs of acute thromboembolic events were noted. Despite all the complications neither the patient nor the family requested de-escalation of therapy with exception of the last two weeks of life.
This case shows an unusual accumulation of major and live-threatening complications in a single patient following LTx. Serious cardiac complications other than ischemic heart disease and arrhythmia are rare in LTR and there are only a few case reports which describe pneumopericardium as a complication in this population.1–4 Neurological complications in the early postoperative phase occur in about 9.2%, the majority included stroke and encephalopathy as the underlying pathology.5 This case shows that maximal invasive therapy by a multidisciplinary team approach may be successful in seemingly hopeless or critical complications after LTx.6 Quality of life may be judged differently by the patient or family than by health professionals.7 In this case the wish for palliative treatment was only requested after strong deterioration of the lung function when the patient became dependent on the help of her family. Both the patient and the family expressed gratitude for the extra time gained by the LTx despite the initial difficulties experienced.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNone.
Conflict of interestNone.