Phenotypic overlap between the two main chronic airway pulmonary diseases, asthma and chronic obstructive pulmonary disease (COPD), has been the subject of debate for decades, and recently the nomenclature of asthma-COPD overlap syndrome (ACOS) was adopted for this condition. The definition of this entity in the literature is, however, very heterogeneous, it is therefore important to define how it applies to Portugal.
MethodsA literature review of ACOS was made in a first phase resulting in the drawing up of a document that was later submitted for discussion among a panel of chronic lung diseases experts, resulting in reflexions about diagnosis, treatment and clinical guidance for ACOS patients.
ResultsThere was a consensus among the experts that the diagnosis of ACOS should be considered in the concomitant presence of: clinical manifestations characteristic of both asthma and COPD, persistent airway obstruction (post-bronchodilator FEV1/FVC<0.7), positive response to bronchodilator test (increase in FEV1 of ≥200mL and ≥12% from baseline) and current or past history of smoking or biomass exposure. In reaching diagnosis, the presence of peripheral eosinophilia (>300eosinophils/¿L or >5% of leukocytes) and previous history of atopy should also be considered. The recommended first line pharmacological treatment in these patients is the ICS/LABA association; if symptomatic control is not achieved or in case of clinical severity, triple therapy with ICS/LABA/LAMA may be used. An effective control of the exposure to risk factors, vaccination, respiratory rehabilitation and treatment of comorbidities is also important.
ConclusionsThe creation of initial guidelines on ACOS, which can be applied in the Portuguese context, has an important role in the generation of a broad nationwide consensus. This will give, in the near future, a far better clinical, functional and epidemiological characterization of ACOS patients, with the ultimate goal of achieving better therapeutic guidance.
Asthma and COPD are chronic lung diseases which are highly prevalent and have significant socio-economic impact.1,2 Data from a recent nationwide study indicate that the current asthma prevalence in the Portuguese population is 6.8%.3 The national prevalence for COPD was estimated in 9.0% and 5.3% in 2 previous studies – in selected age groups (≥40 years old in one study and between 35 and 69 years old in the other); there is also another study carried out in the Lisbon area that showed a prevalence of 14.2% (in patients of 40 years old or more).4,5 Both asthma and COPD affect the airways and are characterized by the presence of bronchial obstruction.1,2 Even though these pathologies are heterogeneous, they usually present quite characteristic clinical symptoms, functional changes and underlying physiopathology, which enables a straightforward diagnosis in a majority of patients.1,2,6 However, there is a growing consensus that typical asthma and COPD characteristics can both exist simultaneously in one patient, especially in those who are older and have a history of smoking.6–8 Data from the INAsma study clearly show that, in Portugal, asthmatic patients smoke in the same proportion as the non-asthmatic and that the passive exposure is even higher in the first group.9 In reality, there are patients with severe asthma, that has evolved over a long period and frequently with smoking habits, that eventually develop fixed airway obstruction, a pattern usually seen in COPD.10,11 On the other hand, a positive bronchodilator test, as seen in many asthma patients, can be found in a significant proportion of COPD patients, although not of same magnitude.12,13 In this context, the concept of ACOS (asthma-COPD overlap syndrome) has been used to describe this set of patients that present concomitant asthma and COPD characteristics. It is important to highlight that in this group of patients, although they show a broad clinical heterogeneity, there are essentially two types of patients: the asthmatic patient that develops ACOS and the COPD patient that presents clinical characteristics of ACOS. It is, thus, important to be aware that in these cases there is an initial distinct physiological base that culminates in an overlap of symptoms, which can have implications for diagnosis and therapy.
The way these patients are characterized by the several entities analysing this issue is very heterogeneous, which makes it difficult to apply the concept of ACOS to the clinical situation in Portugal. In this context, there is a need for a first step towards clarification of concepts applied to the national context, in order to develop, in the near future, a broader ACOS medical consensus. This paper is an indexed literature revision on the subject, complemented by a series of critical reflexions regarding diagnostic criteria, patient identification, therapeutic approaches and guidelines for future clinical investigation.
MethodsA literature review was carried out via the PubMed database by searching for MeSH Terms (“asthma”, “chronic obstructive lung disease”, “overlap syndrome”). Articles between 2006 and 2016 were selected as relevant if they had epidemiological data, diagnostic criteria, clinical symptoms and impact and therapeutic approaches. In a second phase, a working meeting was held with medical experts from the chronic lung diseases field (Pulmonology, Immunoallergology, Family Medicine) where the several topics presented in this paper were discussed and proposals for recommendations on ACOS adapted to the Portuguese context drawn up.
Clinical characterization and impactThe differentiation in terms of respiratory symptoms between asthma and COPD is, in many cases, quite difficult, because there are several areas where they can overlap, making the distinction more complicated. For example, the presence of chronic productive cough is more associated with COPD but can also be present in an asthmatic patient, which leads to a worse prognosis in terms of pulmonary function decline.14 On the other hand, it is also common to have the presence of asthmatic symptoms (occasional dyspnoea, sibilance) in COPD patients.15 In terms of bronchodilator response, the reversibility seen, although typical in asthma, is not exclusive to it, as it is also observed in up to 50% of COPD patients.16 Furthermore, the bronchial hyperresponsiveness, which is present in almost all asthma patients, can also be seen in a significant percentage of COPD cases.17
This clinical diversity leads to the overlap between these two obstructive respiratory diseases. It is possible, however, to isolate characteristics that enable the recognition of a new entity (ACOS) which aggregates features from both (asthma and COPD).
Several studies have analyzed the clinical differences between ACOS, COPD and asthma, showing a higher prevalence of respiratory symptoms in patients with ACOS compared to the other two alone.18,19 In the ACOS group, studies show a more significant exertional dyspnoea (assessed by the mMRC score),20 a higher percentage of sibilance when compared with COPD patients,21 less physical capacity, more exacerbations and lower quality of life.22 In the population-based study (EPI-SCAN21) the authors compared the prevalence of symptoms between COPD, asthma and ACOS patients, observing a higher percentage of dyspnoea in the ACOS group, more sibilance in the ACOS group when compared to the COPD group, and an equal percentage of productive cough in COPD and ACOS. Two other studies (GEIRD and PLATINO18,22) revealed, however, a higher percentage of productive cough in ACOS patients, even when compared to isolated cases of COPD.
On the other hand, the exercise capacity shown by ACOS patients was prospectively analyzed by Fu et al. in a 4-year follow-up study which concluded that the functional decrease in terms of 6-min walk test (6MWT) was lower in these patients compared to the COPD group.23
There is no consensus on the results concerning lung function, although many studies show lower values of FEV1, FVC and FEV1/FVC in patients with ACOS compared to COPD and asthma.18,24 Other studies reveal that there are no significant differences between these groups in this area.23,25,26
In relation to radiological differences between ACOS and COPD, there seems to be a slightly less emphysema expression in the first group as well as a predilection for the upper lung lobes.27
There is a higher degree of consensus between the studies over exacerbations, with a higher rate in ACOS patients.18,19,21 There is also a higher percentage of severe exacerbations and a higher rate of hospitalizations. In a study performed by Brzostek and Kokot a significant rate of recent exacerbations (69% in the last year) was observed.28
In terms of comorbidities, studies point towards a high prevalence in ACOS, especially cardiovascular ones. Some authors have referred to a higher incidence in ACOS patients compared to COPD and asthma but that is not applicable to the whole literature.20,24,28 Miravittles et al. have used the Charlson Comorbidity Index as a mortality prognostic indicator, showing a significantly higher value in ACOS patients.21
Chronic obstructive pulmonary diseases, due to its prevalence and health-resource consumption needs, have a higher economic burden associated with them. Within these, COPD has clearly more burdensome than asthma.29 However, a cost comparative analysis between ACOS and COPD, clearly shows a higher value for the former, mainly because of the higher rate of hospital admission.30
The ACOS-associated mortality rate was addressed in a recent analysis of a multicentric Italian study (SARA study),31 showing no significant differences compared to COPD but much higher than asthma.
Diagnostic criteria/biomarkersClinical criteriaThe identification of patients with ACOS in daily clinical practice is based, in a first phase, on the recognition of certain clinical features of both asthma and COPD being present simultaneously in the same patient, as previously stated.6,7
Proposed recommendation: simultaneous presence of clinical features of asthma and COPD should be considered as a criterion for the diagnosis of ACOS.
Spirometric criteriaThe presence of persistent airflow limitation, defined as post-bronchodilator FEV1/FVC<0.7, is one of the criteria proposed by several authors for the diagnosis of ACOS.18,32,33 The fixed cut-off value of this ratio, although an essential criterion for the diagnosis of COPD, does not help, however, the differentiation between asthma and COPD.2,6
A positive response to the bronchodilator test, usually associated with asthma diagnosis, can also be found in certain patients with COPD.1,16,34 In these, this response tends to present a lower magnitude, may be inconsistent over time and does not necessarily reflect the presence of overlap syndrome.16,34,35 However, since there are certain individuals who only manifest the first symptoms of asthma in adulthood, the presence of a positive response to bronchodilator test is also frequently assumed to be an agreed criterion to be considered for the diagnosis of ACOS, especially if it is a very positive response (increase of 15% and 400mL in FEV1).36,37
Proposed recommendation: there was consensus among experts that the presence of persistent airway obstruction (defined as post-bronchodilator FEV1/FVC<0.7) associated with evidence of a positive response in bronchodilator test (defined as an increase in the value of FEV1 of ≥200mL and ≥12% from baseline) at least in one functional evaluation should be a criterion for the diagnosis of ACOS.
Systemic and airway inflammationAirway inflammation is a common feature of asthma and COPD; in many asthma phenotypes it is predominantly eosinophilic, while in COPD there is a predominant neutrophilia.38 However, in asthmatic smokers or in severe or late-onset asthma, a neutrophilic inflammation has been demonstrated, which is similar to COPD.38,39 On the other hand, peripheral or sputum eosinophilia, as well as the elevation of the fractional exhaled nitric oxide (FENO) and immunoglobulin E (IgE), although generally more often observed in asthmatic patients, have also been demonstrated in certain patients with COPD.1,40–43 In addition, a higher peripheral and sputum eosinophilia have been found in patients with COPD and partial reversibility of airflow limitation44; it was further observed that the presence of eosinophilia in the sputum of COPD patients has been associated with a better response to treatment with inhaled corticosteroids.16 It is further noted that, in asthma, the presence of sputum eosinophilia is a factor that can be determinant for the development of fixed airway obstruction.45 Thus, the applicability of these markers has been subject of study to support the diagnosis of ACOS.43,46
The identification of other systemic inflammation biomarkers that aid the diagnosis and help towards a better classification of patients with obstructive airway diseases has been investigated. Although several markers have been suggested, such as interleukin-6 (IL-6), periostin, C-reactive protein, among others, none of these has been, to date, included as diagnostic criteria, given the lack of evidence to support their applicability, since their role has not been yet completely determined.47–49
Proposed recommendation: peripheral eosinophilia (defined by the presence of >300eosinophils/¿L or >5% of the leukocytes) and elevation of IgE are aspects frequently found in this group of patients, and should be taken into account when considering the diagnosis, although they cannot be used as main diagnostic criteria. Since the determination of sputum eosinophilia is a method that is not widely available in Portugal and the determination of FENO has fallen into disuse, we did not consider recommending their inclusion as diagnostic criteria which could be used in clinical practice. There is no scientific evidence enough to support its use. There is not enough scientific evidence to support the use of other potential serum biomarkers in this context.
Exposure (tobacco and biomass combustion)Smoking has been established as a risk factor for the development of COPD and it accelerates the rate of lung function decline in both asthma and COPD.1,2,50 Additionally, it may be at the bottom of fixed airway obstruction development in asthmatics.1,51 In a similar way, exposure to biomass combustion is also associated with airway obstruction.2,52 Thus, (current or past) smoking habits, as well as a history of exposure to biomass, are generally included as criteria for the diagnosis of ACOS.
Proposed recommendation: the presence of current or past history of smoking or biomass combustion exposure should be considered as a criterion for the diagnosis of ACOS, as this exposure is associated with the development and severity of asthma and COPD.
History of asthma or atopy before 40 years oldThe diagnosis of asthma is most commonly made in childhood, but sometimes it can only be diagnosed in adulthood.1 Additionally, asthma, by itself, is a risk factor for COPD development.53 On the other hand, atopy is assumed to be a risk factor commonly associated with asthma, but can also be found in a significant percentage of patients with COPD and may be a risk factor for development of COPD.1,54–56 Thus, in most publications, for patients diagnosed with COPD, these criteria have been taken into consideration for diagnosis of ACOS.
Proposed recommendation: the presence of a previous history of atopy is an aspect often found in this group of patients, so it should be taken into account when considering the diagnosis, although it cannot be assumed as a diagnostic criterion. It was not considered important to establish an age limit that should be taken into account or applied as a criterion.
Bronchial hyperresponsivenessIt has been shown that the presence of bronchial hyperresponsiveness, even though it may be found asymptomatically in the general population, is associated with an increased risk of asthma and COPD, and might in both cases be a marker of more severe, more symptomatic disease and a greater decline in lung function.57,58 In fact, bronchial hyperresponsiveness, present in virtually all asthmatic patients, may also be found in 60–90% of patients with COPD, and in these it may be associated with more symptoms and greater severity of obstruction.17,59
Proposed recommendation: since the presence of bronchial hyperresponsiveness is expected in asthmatic patients and bearing in mind that it can be detected in a very high proportion of COPD patients, the presence of this aspect, which has a low specificity, was not considered relevant for the diagnosis.
DefinitionSeveral study groups have published highly diverse proposals for definitions and diagnostic criteria for ACOS, most of these recommendations originating from expert opinion consensus.47,60–62 The description proposed by the joint project of GOLD and GINA characterizes ACOS as the presence of persistent airflow limitation with several characteristics usually associated with asthma and several characteristics usually associated with COPD.6 This definition is vague and the diagnosis is based on the balance of attributes taken from a checklist with typical asthma and COPD aspects.
In fact, ACOS is still poorly characterized, both in terms of general risk factors and pathophysiology, and in terms of clinical symptoms, treatment response and prognosis. This is largely due to the fact that patients who meet criteria compatible with a possible diagnosis of ACOS are usually excluded from clinical trials targeting COPD or asthma.
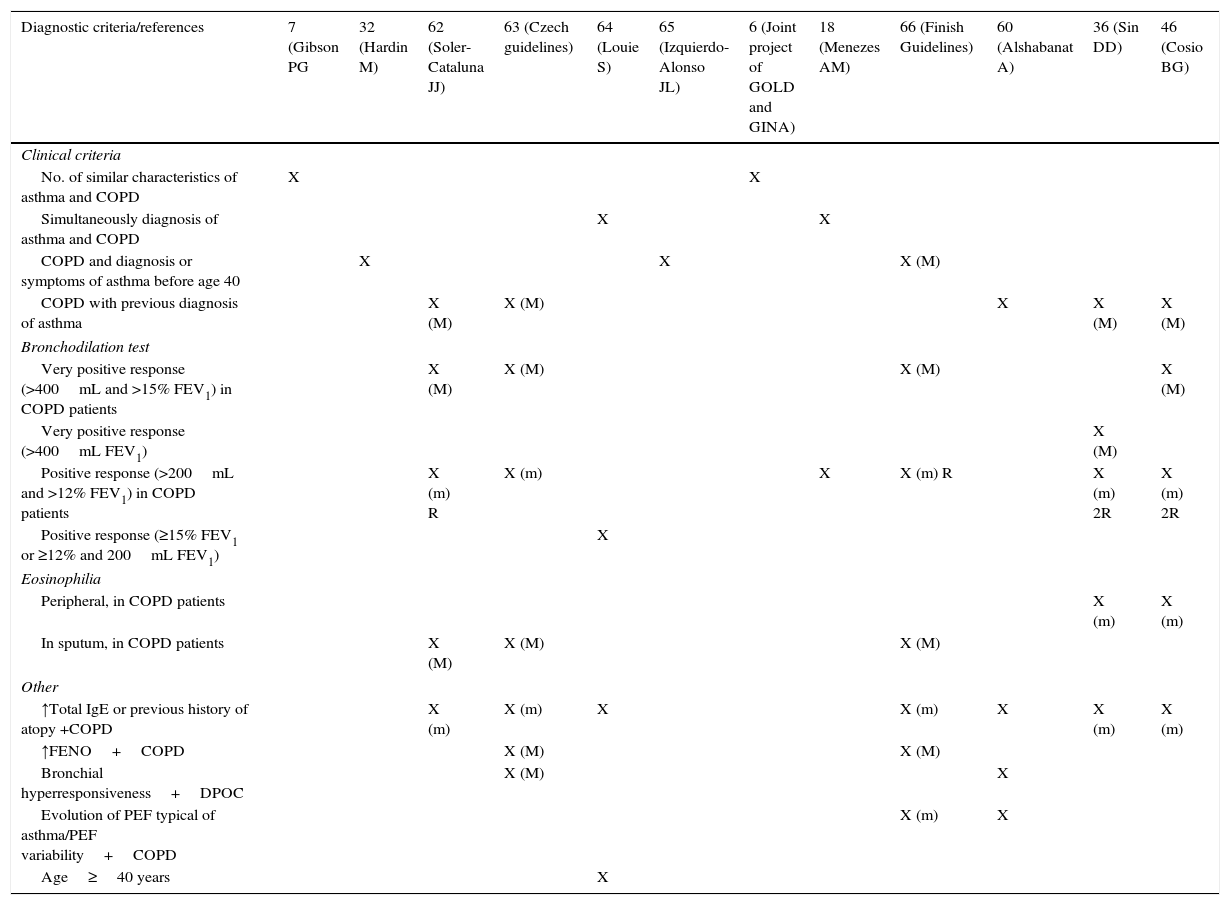
Table 1 summarizes the main definitions and diagnostic criteria proposed by several authors.
Main definitions and diagnostic criteria proposed.
| Diagnostic criteria/references | 7 (Gibson PG | 32 (Hardin M) | 62 (Soler-Cataluna JJ) | 63 (Czech guidelines) | 64 (Louie S) | 65 (Izquierdo-Alonso JL) | 6 (Joint project of GOLD and GINA) | 18 (Menezes AM) | 66 (Finish Guidelines) | 60 (Alshabanat A) | 36 (Sin DD) | 46 (Cosio BG) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical criteria | ||||||||||||
| No. of similar characteristics of asthma and COPD | X | X | ||||||||||
| Simultaneously diagnosis of asthma and COPD | X | X | ||||||||||
| COPD and diagnosis or symptoms of asthma before age 40 | X | X | X (M) | |||||||||
| COPD with previous diagnosis of asthma | X (M) | X (M) | X | X (M) | X (M) | |||||||
| Bronchodilation test | ||||||||||||
| Very positive response (>400mL and >15% FEV1) in COPD patients | X (M) | X (M) | X (M) | X (M) | ||||||||
| Very positive response (>400mL FEV1) | X (M) | |||||||||||
| Positive response (>200mL and >12% FEV1) in COPD patients | X (m) R | X (m) | X | X (m) R | X (m) 2R | X (m) 2R | ||||||
| Positive response (≥15% FEV1 or ≥12% and 200mL FEV1) | X | |||||||||||
| Eosinophilia | ||||||||||||
| Peripheral, in COPD patients | X (m) | X (m) | ||||||||||
| In sputum, in COPD patients | X (M) | X (M) | X (M) | |||||||||
| Other | ||||||||||||
| ↑Total IgE or previous history of atopy +COPD | X (m) | X (m) | X | X (m) | X | X (m) | X (m) | |||||
| ↑FENO+COPD | X (M) | X (M) | ||||||||||
| Bronchial hyperresponsiveness+DPOC | X (M) | X | ||||||||||
| Evolution of PEF typical of asthma/PEF variability+COPD | X (m) | X | ||||||||||
| Age≥40 years | X | |||||||||||
X, indicates the references that include the criteria; M, considered a major criterion; m, considered a minor criterion; R, in repeated assessments; 2R, in at least two assessments.
Therefore, although there is no agreed, established and validated definition for ACOS, this entity is widely recognized in clinical practice as an individualized phenotype demarcated from the spectrum of chronic obstructive airways disease.67 In addition, the identification/recognition of this phenotype of chronic obstructive respiratory disease may influence the prognostic and therapeutic approach. Thus, it is really necessary to establish a consensus, based on a review of the available literature and professional experience, to standardize the diagnosis of ACOS and outline an approach strategy for this group of patients, for whom randomized controlled clinical trials (RCTs) are still missing.
Proposed recommendation: the diagnosis of ACOS should be considered in the concomitant presence of:
- 1)
simultaneous clinical manifestations characteristic of both asthma and COPD
- 2)
persistent airway obstruction, defined as post-bronchodilator FEV1/FVC<0.7, evaluated in a period of clinical stability
- 3)
positive response in bronchodilator test, defined by an increase in the value of FEV1 of ≥200mL and ≥12% from baseline
- 4)
current or past history of smoking or exposure to biomass combustion
As aspects that are usually present in this group of patients and that can be taken into account in the diagnostic consideration, we highlight peripheral eosinophilia (>300eosinophils/¿L or >5% of leukocytes) and previous history of atopy. In annex 1, a representation of the proposed algorithm is presented.
PrevalenceViews on the prevalence of ACOS vary greatly among the published studies, reflecting the different diagnostic criteria applied in each, as well as the different populations analyzed.68–76
In asthmatic patients, prevalence of ACOS has been reported as ranging between 13 and 30%.22,77–79 However, when broader criteria are used and subpopulations of older patients are analyzed, the prevalence recorded is higher; for example, in a subgroup of asthmatic patients older than 65 years, a prevalence of 61% was found.22
Within the group of patients with COPD, the estimated prevalence of ACOS is also varies greatly across studies, with values ranging between 9% and 55%.18,65,77,80–82
In the population-based study PLATINUM, the prevalence of ACOS was 2%, and the prevalence of asthma and COPD was 2% and 12%, respectively.18 In other similar studies, a prevalence of ACOS in the general population ranging between 2 and 5% was found, with increasing prevalence associated with an increase of the age group being analyzed.22,83
Due to lack of studies to date, ACOS prevalence data relating to Portugal are not known
ApproachTreatmentSimilar to the other chronic obstructive pulmonary diseases, the therapeutic approach to ACOS patients always starts with a risk factor exposure control, in which we highlight smoking, exposure to biomass, allergens exposure, anti-infectious prevention, among many others. It is important to have a clinical based approach, balanced with the presence of comorbidities and further assessment (lung function, eosinophilia, among others).
In terms of pharmacological therapy, the clinical evidence in ACOS is limited, because the majority of these patients are systematically excluded from most of the clinical COPD and asthma pharmacological clinical trials. Only three studies (one with the use of LAMA84 and the other two with oral corticosteroids85,86) were performed specifically in this group of patients. The majority of the consensus documents points, however, towards an important role of bronchodilators with LABA, isolated or in combination with LAMA, always associated with ICS.6,63,66,87 The use of ICS/LABA as a first line of therapy is recommended by the majority of the consensus.
The use of LABA alone in asthma patients has been associated with poor disease control, increase of its severity and mortality, and therefore its use is contraindicated in asthma patients.88 Although this fact has not been established in ACOS, the majority of the guidelines extrapolate this consideration into this group.
The use of ICS in ACOS patients has been revealed as beneficial when compared to its use in COPD, resulting in an improvement in FEV1.89 The ICS dose used can be adjusted to each patient, depending on their symptoms and smoking habits.64,90,91
Triple therapy with ICS/LABA/LAMA has shown an exacerbation reduction in COPD patients92 but this fact has yet to be proven in ACOS. However, most of the consensus documents consistently point to the use of this approach in non-controlled patients with ICS/LABA.
New therapies have begun to be studied in ACOS patients, such as the use of the monoclonal antibody anti-IgE, omalizumab, which seems to show promising results in terms of symptomatic improvement and exacerbations reduction.93,94 Other specific therapies focused on the relevant role of eosinophils (anti IL-5, anti IL-13 and anti IL-33 drugs), treatments regarding the neutrophilic expression (macrolides, p38 mitogenactivated protein kinase inhibitors, anti IL-1 and anti IL-17 antibodies, phosphodiesterase 4 inhibitors) could play a significant role on the prognosis of ACOS patients.95
As referred to above, the appropriate treatment of the frequent comorbidities present in these patients is crucial, as well as an effective vaccination coverage and the implementation of a pulmonary rehabilitation programme, especially in patients with a higher COPD burden, which in more advanced stages, determines the prognosis of patients with ACOS characteristics. Moreover, it is necessary to verify in a consistent and regular way the inhalation technique, reinforcing the importance of the inhaled therapy adherence.
Proposed recommendations:
- -
ICS/LABA as first line therapy. In patients which are not controlled or whose clinical severity justifies, a triple therapy with ICS/LABA/LAMA should be used.
- -
Non-pharmacological therapy such as pulmonary rehabilitation should be done in ACOS patients with uncontrolled symptomatology (frequent exacerbations).
- -
Comorbidities treatment should be optimized for a better control of the lung disease.
- -
Risk factors exposure control (smoking, biomass, allergens exposure) and vaccination coverage (influenza and anti-pneumococcal).
The best way forward for these patients in terms of health care is still not completely established, however, the GINA and GOLD recommendation document reveals some guidance such as: ACOS patients should be referred to a specialist if they present persistent or uncontrolled symptoms and/or common exacerbations, if there is a diagnostic uncertainty, atypical symptoms/signs, or important comorbidities.6
Proposed recommendations:
- -
The patient with ACOS should be given a specialized hospital appointment if control of symptoms has not been achieved or if there is a diagnostic uncertainty. If clinical stability is achieved in a consistent way, the patient's follow-up can be performed by the family doctor.
- -
The frequency of the follow-up of these patients is going to depend on their clinical stability and/or severity. However, given the characteristics shown by these patients, a medical observation for symptoms control on, at least, a twice a year basis is recommended, as well as a spirometric evaluation with a bronchodilator test at least once a year. To help the assessment of symptoms control, the mMRC dyspnoea scale and, especially, in patients with a higher asthmatic component, the CARAT questionnaire (although not validated in ACOS) should be used.
This document sets forward the heterogeneity of diagnosis that still exists in this area, which underlines its importance as a first stage in the examination of this field. It seems clear there is a group of patients who share characteristics that cross the COPD and asthma spectrum, it is therefore crucial to achieve a more accurate identification of these patients, enabling a more effective therapeutic approach. In the future this characterization of ACOS patients will provide for the development of national prevalence studies and the evaluation of the impact of different pharmacological and non-pharmacological therapies, which will complement our knowledge of this entity and optimize treatment strategies.
This document constitutes a first step towards what might become a nationwide Portuguese consensus in relation to ACOS, which would strengthen the medical community's vision on this subject.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank the participation, as members of the Advisory Board, and contribution in the face-to-face meeting for discussion and revision of the theme to the physicians: Marta Drummond, MD, PhD, Mafalda van Zeller, MD, Margarida Redondo, MD, Eurico Silva, MD, Rui Costa, MD, Jaime Correia de Sousa, MD, PhD, Ana Todo Bom, MD, PhD, Tiago Alfaro, MD, Bugalho de Almeida, MD, PhD, Manuel Branco Ferreira, MD, PhD, Sandra André, MD and Filipa Todo Bom, MD.
It is recognized the support in the form of Educational Grant from Mundipharma Pharmaceuticals Ltd.